FIGURE 4.
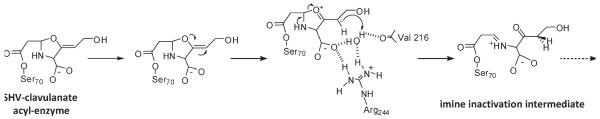
Proposed reaction mechanism for SHV-1 and clavulanate showing the formation of the imine inactivation intermediate. Scheme shows the contribution of Arg244 in the coordination of the water molecule hypothesized to donate a proton for C2 double bond saturation and subsequent secondary ring-opening. In SHV-1, it is postulated that the water molecule is recruited or relocated into the active site with the binding of clavulanate (13). The crystal structure of TEM Asn276Asp (PDB entry 1CK3) reveals that this crucial water is missing, offering an explanation for the IR phenotype as protonation of clavulanate would be impaired, and thus lead to more ready hydrolysis of the inhibitor than in TEM-1, where the water is clearly refined (19).
