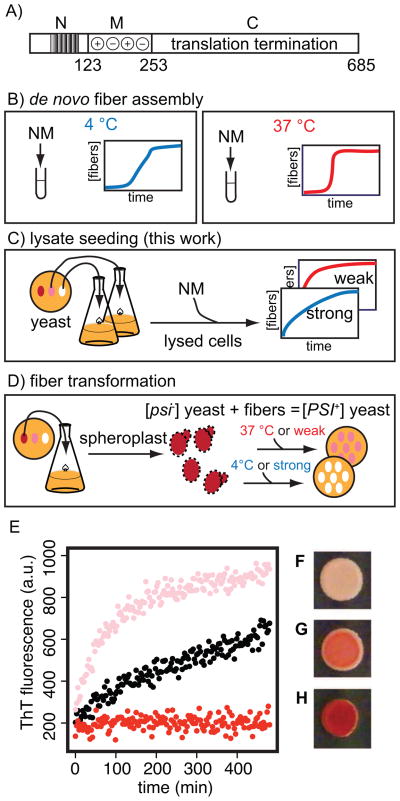
Figure 1.
a) Schematic diagram of the domains of the Sup35 protein. b) After a lag phase, purified NM spontaneously forms fibers in vitro. c) Fiber formation can be templated from yeast cell lysates. The character of the fibers formed matches the character of the fibers in the yeast lysate used. d) Introduction of NM fibers into spheroplasted [psi−] yeast confers the [PSI+] state in a strain specific manner. Control of lysate-templated prion fibers. e) Fiber formation as monitored by Thioflavin T fluorescence for lysate seeded NM maintains distinct kinetic character for strong (black) and weak (pink) [PSI+] strains. Lysates from [psi−] cells (red) do not template NM amyloid assembly. Introduction of lysate-templated strong fibers (f), weak fibers (g) and unpolymerized NM (h) into [psi−] yeast cells confers expected [PSI+] phenotypes. All 13 [PSI+] colonies resulting from transformation with strong prion fibers had strong phenotypes and all 3 [PSI+] colonies resulting from transformation with weak prion fibers had weak phenotypes.