Abstract
Ormeloxifene is a non-steroidal Selective Estrogen Receptor Modulator (SERM) that is used as an oral contraceptive. Recent studies have shown its potent anti-cancer activities in breast, head and neck, and chronic myeloid leukemia cells. Several in vivo and clinical studies have reported that ormeloxifene possesses an excellent therapeutic index and has been well-tolerated, without any haematological, biochemical or histopathological toxicity, even with chronic administration. A reasonably long period of time and an enormous financial commitment are required to develop a lead compound into a clinically approved anti-cancer drug. For these reasons and to circumvent these obstacles, ormeloxifene is a promising candidate on a fast track for the development or repurposing established drugs as anti-cancer agents for cancer treatment. The current review summarizes recent findings on ormeloxifene as an anti-cancer agent and future prospects of this clinically safe pharmacophore.
Keywords: Breast cancer, cancer, chemo-resistance, chronic myeloid leukemia, contraceptive, head and neck squamous cell carcinoma, ormeloxifene, ovarian cancer, prostate cancer, SERM
INTRODUCTION
Targeting the estrogen receptor (ER) by a Selective Estrogen Receptor Modulator (SERM), tamoxifen, is the oldest form of molecular-targeted therapy in breast cancer treatment. However, major limitations associated with current SERMs include their inadequate specificity, loss of activity and early onset of resistance. Therefore, with an aim to increase specificity and to circumvent difficulties associated with the treatment of cancers refractory to drug(s), several classes of SERMs were synthesized in recent years. One such SERM, ormeloxifene (also known as centchroman [3,4-trans-2,2-dimethyl-3-phenyl-4-p-(b-pyrrolidinoethoxy) phenyl-7-methoxy chroman]) (Fig. 1A), was synthesized by the Central Drug Research Institute (CDRI), Lucknow, India. Because it is free of the side effects commonly associated with estrogen and progestin based oral contraceptives, this drug has been developed into an effective, safe and easy to use oral contraceptive. It is approved for use in India and multiple other countries including Thailand, Bangladesh, Russia, New Zealand, United Kingdom and some North and South American countries [1]. Like a classical SERM, ormeloxifene suppresses the functions of the ER in the reproductive organs (ovaries, uterus) and breast, whereas it stimulates the ER in other organs like the bones [1, 2].
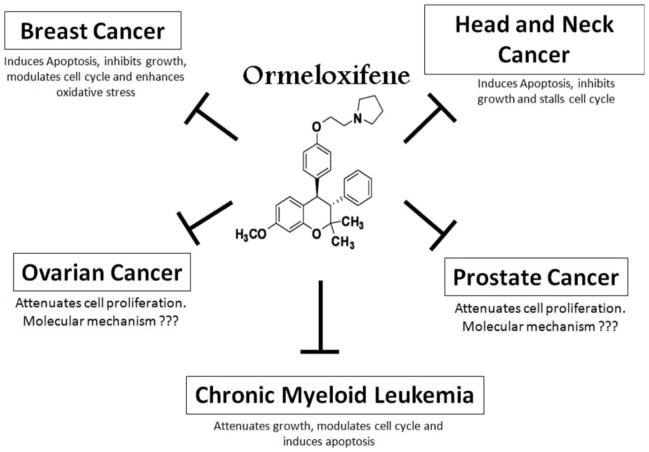
Fig. 1. Ormeloxifen efficiently attenuates the growth of multiple types of cancer cells.
The structure of ormeloxifene is shown in the center. Ormeloxifene inhibits the growth of breast, head and neck cancer and chronic myeloid leukemia via the modulation of multiple pathways. It also attenuates the growth of prostate and ovarian cancer cell growth through other unknown mechanisms.
Ormeloxifene was introduced over 20 years ago as the first non-steroidal contraceptive in India under the trade names Saheli, Novex and Novex DS. Its contraceptive action appears to mainly rely on the ability to prevent embryonic implantation in the uterine wall by suppressing endometrial proliferation and decidualization [3]. Ormeloxifene has many advantages as a contraceptive drug including 1) this is a once a week oral pill (30 mg dose, weekly), 2) it has long serum half-life, 3) it has very few to no side effects since it does not affect ovulation or the levels of female hormone, and 4) it provides an opportunity for effective and efficient reversal of fertility upon discontinuation of the drug. In addition, the drug is cost effective, highly stable and does not require any specific storage conditions. These advantages of ormeloxifene have established its use as a highly effective birth control pill (contraceptive or morning-after pill) in women [1].
Recently, ormeloxifene has also been reported as a potent anti-neoplastic agent in various cancers [1, 4–8]. In vitro and in vivo studies have demonstrated several advantages of ormeloxifene as a pharmacophore, including extended serum half-life, favorable pharmacokinetic and pharmacodynamic properties, absence of severe toxicity, and cost effectiveness. Therefore, ormeloxifene can be developed as a potent therapeutic anti-cancer agent [1, 4–9]. This review summarizes recent findings on the role of ormeloxifene as an anti-cancer agent. We also outline some future directions for developing this promising pharmacophore as an anti-cancer agent for the treatment and management of cancers.
CHEMISTRY, PHARMACOKINETICS AND METABOLISMS OF ORMELOXIFENE
Chemistry and Synthesis
Ormeloxifene (C30H35O3N.HCl) is a white crystalline substance (MW 493.5 Da) with a melting point of 165–166°C. It is soluble in organic solvents like chloroform, acetone, methanol, and ethanol, but is almost insoluble in water. Ormeloxifene is a highly stable compound under normal storage conditions with no marked change in color, appearance, purity, or biological efficacy. At room temperature, ormeloxifene has been found to be stable and retain its biological activity and characteristics for at least 3 years when stored in glass containers or aluminum strips [1, 10, 11]. The chemical synthesis of ormeloxifene can be carried out through multiple processes [1, 10]. A primary mode of synthesis involves the Grignard reaction of cis-3-phenyl- 4-p-acetoxyphenyl-7-methoxy-3,4-dihydrocoumarin with methylmagnesium iodide in tetrahydrofuran (THF) to produce erythro-2-methyl-3-phenyl-4-(p-hydroxyphenyl)-4-(2-hydroxy-4-methoxyphenyl)-butan-2-ol. This compound upon cyclization with polyphosphoric acid (PPA) at 75–80°C yields cis-2,2-dimethyl-3-phenyl-4-p-hydroxyphe-nyl-7-methoxychroman [1, 2]. The isomerization of this compound with n-butyl lithium in DMSO produces an equimolar mixture of the D and the L enantiomer of ormeloxifene [12].
Pharmacokinetics and Bioavailability
Ormeloxifene is a lipophylic molecule that possesses highly favorable pharmacokinetic and pharmacodynamic properties. This drug demonstrates low binding affinity to plasma albumin (Kd value of 13.90× 10−6) and does not interact with steroids like testosterone, cortisol, estradiol and progesterone [13–16]. It is therefore predicted to not interfere with the binding of steroids to specific steroid binding plasma proteins [17–19]. Additionally, while ormeloxifene is a selective modulator of estrogen receptor(s), it has been shown to neither compete with nonsteroidal estrogen agonists like diethylstilbestrol nor with estrogen antagonists like tamoxifen or nafoxidene [19].
Ormeloxifene is quickly metabolized by the liver and excreted from the body primarily via feces [20]. Information on ormeloxifene metabolism, organ retention and circulating levels has been primarily obtained from animal studies [21]. These studies reveal that well-perfused organs like the liver, lungs and spleen tend to retain more ormeloxifene than the less perfused organs like the pancreas and muscle. The active metabolite of ormeloxifene is 7-desmethylated ormeloxifene, which is rapidly formed within 1h of ormeloxifene administration and usually peaks between 8–24h [22]. While the liver is one of the first sites of drug metabolism of orally administrated ormeloxifene [21], high concentrations of the drug and its active metabolite are also found in the spleen, lungs, uterus and adipose tissues. Interestingly, the accumulation of the drug or its metabolite is always higher in tissue than in plasma and the concentration of ormeloxifene in tissue or plasma is invariably higher than its major metabolite, 7-desmethylated ormeloxifene [22]. In adult rats treated with a single oral dose of 12.5 mg/kg ormeloxifene, the maximum plasma concentration of 210 ng/ml was reached after 12h. The overall half-life of ormeloxifene or desmethylated ormeloxifene in adult female rats is about 24h and 36h, respectively.
In human clinical trials, healthy female volunteers were administered with either a single oral dose of 60 mg or 30 mg ormeloxifene. The overall half-life of the drug was detected to be around 168h [23]. Therefore, rats and other lower animals appear to metabolize ormeloxifene faster than humans. The maximum serum concentration (Cmax) of ormeloxifene in humans was dose dependent (Cmax of 55.53 ± 15.43 ng/ml for 30 mg dose and Cmax of 122.57 ± 6.25 ng/ml for 60 mg dose) and was reached within 4h–6h [23]. Similar Cmax values were also detected in breast cancer patients treated with either 30 mg, twice a week for 12 weeks (Cmax 54.98 ± 14.19 ng/ml) or 60 mg of ormeloxifene on alternate days for 1 month or 1 year (Cmax 135 ± 15.5 ng/ml). The serum concentrations of the drug and its half-life in nursing and non-lactating mothers were quite similar. Interestingly, about 2.5% of the ormeloxifene dose was found to be excreted in milk [24]. However, the amount consumed by a suckling child is thought unlikely to be of any physiological concern [24, 25]. Multiple repeated dosing in adult female volunteers revealed no significant difference in either amount of drug accumulated or the time required for maximal accumulation [26]. It has also been demonstrated that ormeloxifene exhibits strong and long lasting estrogen antagonistic and weak estrogenic activities in a number of different types of assays. While ormeloxifene has also been shown to inhibit progesterone action in some bioassays, it does not possess any androgenic or anti-androgenic activities [15, 16]. The mode of its anti-progesterone action is unknown since ormeloxifene neither affects progesterone expression nor progesterone receptor synthesis or receptor-ligand binding kinetics [3, 27, 28]. At contraceptive dose, ormeloxifene has no affect on the hypothalamo-pituitary-ovarian axis. At much higher doses (ten times the contraceptive dose), ormeloxifene may alter the hypothalamus-pituitary-ovarian response as shown in rats [29]. This is thought to occur as a result of the anti-estrogenic effect of ormeloxifene on the hypothalamus and its agnostic action on the pituitary gland [1, 30]. These data suggest that ormeloxifene is a clinically safe drug, with highly favorable pharmacokinetic and pharmacodynamic properties.
ORMELOXIFENE AS AN ANTI-CANCER AGENT
The ability of ormeloxifene to inhibit rapid cell proliferation in the endometrium during embryonic implantation along with its favorable bioavailability, stability and safety in humans makes it an attractive repurposing molecule for controlling undesired rapid cell proliferation such as endometriosis and cancerous conditions. Ormeloxifene has been shown to prevent cancers of breast and uterus, probably due to its potent estrogen antagonistic activity [1, 2]. Additionally, it also stimulates the formation of new bone mass, perhaps due to the weak estrogenic activity of this drug in bones [1, 2]. Estrogen agonist and antagonists function through estrogen receptors, which play an important role in the normal development and function of reproductive tissues as well as non-reproductive tissues (lungs, colon, prostate, and cardiovascular system) [31, 32]. Therefore, compounds that modulate estrogen receptor functions have been used for the treatment of many cancers, like breast cancer [32]. In this section, we will review the role of ormeloxifene as an anti-neoplastic agent against different types of cancers (Fig. 1).
Ormeloxifene and Breast Cancer
ERα and ERβ are the two main types of receptors involved in ER mediated signaling. [22]. Upon ligand binding, the receptors dimerize and translocate to the nucleus, to regulate the expression of a multitude of genes. In addition, estrogen receptors also regulate non-genomic signaling by activating specific signaling pathways such as Protein Kinase C (PKC), AKT pathways, etc., to regulate various functions within the cell. Therefore, dysregulation of ER signaling is associated with initiation and progression of several cancers, including breast cancer [33]. ERα and ERβ appear to have opposite effects on cell proliferation, apoptosis and motility [33]. The mitogenic properties of ERα play an important role in the proliferation of breast cancer cells and poor disease prognosis [31, 32]. In fact, over 70% of tissue specimens from breast cancer patients exhibit overexpression of ERα. ERβ on the other hand appears to counteract the proliferative and malignant effect of ERα by modulating the expression of many ERα regulated genes [33]. The basal level of ERβ is downregulated in invasive breast cancer. Lowered expression of ERβ is associated with increased invasion in both breast cancer and prostate cancer. Therefore, compounds that can selectively modulate the functions of the ERα signaling may aid in the treatment of breast cancer. Ormeloxifene primarily functions as an estrogen antagonist in many organs including breast tissue. It interacts with both ER subtypes, demonstrating more selectivity and higher affinity towards ERα (8.8%) as compared to ERβ (3%) [34]. This anti-estrogen role of ormeloxifene makes it a choice anti-cancer agent for the treatment of breast cancers where ER functions are up regulated. In addition, similar to many SERMs, ormeloxifene also modulates various other signaling pathways independent of ER expression to regulate cancer cell growth.
The first in vivo use of ormeloxifene as an anti-neoplastic drug on advanced stage breast cancer patients, non-responsive to conventional therapy was reported by Mishra et al. (1989) [3]. In this clinical trial, breast cancer patients were treated with ormeloxifene (60 mg, three times a week) for 4–6 weeks. About 38.5% of breast cancer patients responded to the ormeloxifene therapy. In this clinical trial, ormeloxifene showed relatively better anti-breast cancer activity in older females patients (peri and post menopausal) compared to younger (pre-menopausal) patients. The responses to ormeloxifene treatment were more promising for bone, pulmonary, soft tissue, skin, and lymph-node metastases than for visceral metastases. However, there was no correlation between the number of lesions or estrogen receptor positivity and response to ormeloxifene therapy [3].
Molecular mechanistic studies with this compound have demonstrated that ormeloxifene induces caspase and mitochondrial-dependent apoptosis in breast cancer cells [5, 6]. In ER+ (MCF-7 cells) and ER− (MDA-MB231) breast cancer cell line models, ormeloxifene efficiently inhibited cell proliferation at concentrations similar to tamoxifene. Ormeloxifene, however, was more effective against ER(+) MCF-7 cells than ER(−) MDA-MB231 [5–6] (Fig. 2). Interestingly, ormeloxifene was also found to induce apoptosis at very low concentrations by depolarizing the mitochondrial membrane potential in the cell lines. In addition, ormeloxifene arrested breast cancer cells at the G0/G1 cell cycle phase [5, 6]. This effect correlated with the enhanced expression of cell cycle regulators like p21Waf1/Cip1and p27Kip1 and down regulation of Cyclin-D1 and Cyclin-E expression [6]. Since p21Waf1/Cip1 expression is usually controlled transcriptionally and post-transcriptionally by the p53-dependent/independent pathway [35], both cell types (MCF-7; p53+ and MDA-MB231; p53−) show p21Waf1/Cip1 activation after ormeloxifene treatment leading to cell cycle arrest. Recently it has been reported that ormeloxifene mediated apoptosis involves a cross talk between the extrinsic/intrinsic pathways and the modulation of the redox system in breast cancer cells [5]. Additionally, the efficacy of ormeloxifene in combination with sensitizing agents such as cucurmin and resveratrol has also been investigated [36]. Phytochemicals like curcumin and resveratrol are known to sensitize cancer cells to cytotoxic drugs, enhancing the cellular response to drugs and thereby minimizing side effects [37]. A recent study showed that resveratrol and curcumin sensitized breast cancer cells to ormeloxifene. In combination with resveratrol or curcumin, relatively much lower concentrations of ormeloxifene induced apoptosis in breast cancer cells [36]. These phytochemicals increased pro–apoptotic JNK/p38 signaling and depolarized mitochondrial membrane potential to enhance ormeloxifene mediated apoptosis via activation of caspase-9/caspase-3 and alterations of Bax/Bcl-2 protein ratios [36]. Moreover, the efficacy of ormeloxifene in combination with glycine soya, a dietary estrogen-like compound, was examined for reducing breast cancer in a 7,12-dimethylbenz-[a]anthracene (DMBA)-induced rat mammary tumor model system [38]. In this in vivo study, DMBA-induced tumor bearing mice were fed with either ormeloxifene (5 mg/kg body weight) or glycine soya (3× 104 mg/per kg body weight) alone or in combination for 5 weeks. The combination of ormeloxifene and glycine soya produced very high (98.6%) tumor regression compared to control groups. These results suggest the remarkable potential of ormeloxifene combinatorial anti-cancer therapy [38]. These data also demonstrate that the anti-cancer activity displayed by ormeloxifene involves the modulation of both ER dependent and ER independent pathways. However, detailed mechanistic studies are warranted to precisely understand the molecular basis of its anti-cancer action.
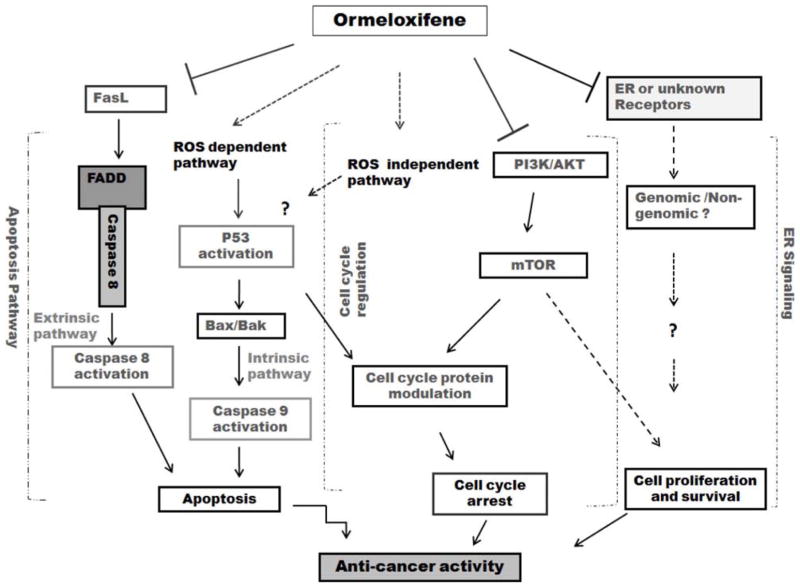
Fig. 2. Cellular functions modulated by ormeloxifene.
Ormeloxifene inhibits cell proliferation and cell cycle progression, while enhancing apoptosis and oxidative stress to inhibit cancer cell growth. The solid arrows represent functions that have been demonstrated in various cancer cell lines. The dotted arrows indicate proposed functions that might be modulated by ormeloxifene.
Ormeloxifen and Prostate Cancer
Prostate cancer is the second deadliest cancer in males after lung cancer [39]. The prostate contains ER in both the stroma and epithelium. Both animal models and human epidemiologic studies have implicated estrogen as an initiator of prostate cancer. In aging males, prostate cancer occurs in an environment of rising estrogen and decreasing androgen levels [40]. Therefore, SERM can be used for prostate cancer treatment by selective inhibition of ER. A few investigations with tamoxifen alone or in combination with other anti-cancer agents (doxorubicin, 5-fluorouracil, leucovorin, mifepriston, vinblastin and quercetin) have been reported [41–43]. Rossi et al. (2011) also investigated the use of another SERM, raloxifene, for the inhibition of prostate cancer cell growth expressing varying levels of ERα and ERβ [44]. Raloxifene efficiently induced cell death and inhibited proliferation of prostate cancer cells via modulation of multiple signaling pathways [44]. However, the use of ormeloxifene as an agent to treat prostate cancer has not been reported yet. Preliminary work from our group suggests that ormeloxifene inhibits growth of androgen dependent and androgen independent prostate cancer cells via inducing apoptosis. Studies also suggest that ormeloxifene attenuates certain key oncogenic pathways to inhibit growth of prostate cancer cells (unpublished results, personal communication). Continued investigations, however, are necessary to elucidate precise molecular mechanisms of its anti-cancer action in clinically relevant prostate cancer cell lines and animal models.
Ormeloxifene and Head and Neck Cancer
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer in the world and approximately 50,000 new cases will be diagnosed in 2012 in the US alone [39]. Currently, no strategies are available for the treatment of advanced stage HNSCC [45]. The role of ER in HNSCC is controversial. While some previous investigations have shown little to no expression of ER in HNSCC [46], many recent investigations attribute a significant role for ER in enhancing cell proliferation and migration in HNSCC [47, 48]. Since ormeloxifene exerts its cytotoxicity via ER dependent and ER independent pathways in cancer cells, Srivastava et al. (2011) investigated for the first time its potential utilization for the treatment of HNSCC [8]. Ormeloxifene very effectively inhibited growth of multiple HNSCC cell lines by inducing apoptotic cell death through the activation of Caspase 3. The aberrant activation of the PI3K/AKT pathway is often seen in HNSCC and therefore, strategies to target this pathway represent one of the promising cancer prevention and therapeutic modalities. Interestingly, ormeloxifene treatment inhibited the phosphorylation of AKT and thereby attenuated downstream AKT signaling. This in turn suppressed the AKT/mTOR, STAT3 signaling pathway and enhanced the expression of p21 and p27 proteins that are associated with cell cycle progression [8]. This resulted in the stalling of cell cycle progression, inhibition of cell proliferation and cell survival, and enhanced apoptosis of HNSCC cells. Although these in vitro results are very promising, further preclinical and clinical studies are warranted to determine its use as an anti-cancer drug in humans.
Ormeloxifene and Ovarian Cancer
Ovarian cancer is an endocrine malignancy with estrogen receptors being expressed in 65% of ovarian cancer patients [49]. Some data suggest that hormonal therapies may have an effect on ovarian cancer in palliative settings [50]. The use of SERMs as anti-neoplastic agent for the treatment of ovarian cancer is controversial [50, 51]. Ovarian carcinoma has been associated with prolonged use of tamoxifen in patients with a previous history of breast cancer [50]. However, a recent in vivo study demonstrated that tamoxifen induced apoptosis and suppresses tumor growth in nude mice in hormonal-dependent ovarian cancer cells [52]. Additionally, tamoxifen in combination with other agents, chemo-sensitized ovarian cancer cells resistant to Pertuzumab [53, 54]. In combination with Gefitinib, it modulated COX-2 and ER expression in ovarectomized mouse uterus. Tamoxifen-loaded nanoparticles were found to circumvent drug resistance in ovarian adenocarcinoma by enhancing intracellular ceramide [55]. Unfortunately, no published work has examined the role of ormeloxifene for the management of ovarian cancer. Preliminary in vitro results, however, suggest that ormeloxifene is a more potent anti-neoplastic agent compared to tamoxifen, especially in ER expressing ovarian cancer cells (unpublished results). Interestingly, ormeloxifene seems to work both as a classical SERM as well as a genotoxic agent in patient derived primary ovarian cancer cells (unpublished results). Currently, the molecular basis of the inhibitory role of ormeloxifene in ovarian cancer is being investigated using both in vitro and in vivo systems. Results from these studies would provide valuable information to evaluate the efficacy of this drug for the treatment of ovarian cancer in clinical settings.
Ormeloxifene in Chronic Myeloid Leukemia
Ormeloxifene has demonstrated excellent anti-proliferative activity not only in several solid tumors, but also in hematological tumors like chronic myeloid leukemia (CML) [56]. Ormeloxifene induced a concentration dependent increase in apoptosis in multiple CML cells lines (U937, HL60, and K562), with the most prominent effect in K562 CML cells [56]. Ormeloxifene arrested the cells in G0–G1 growth phase and induced ERK mediated apoptosis in CML K562 cells. Proteomic analysis of ormeloxifene treated K562 cells using 2-D gel electrophoresis and mass spectrometer revealed 57% of the differentially expressed proteins to be involved in apoptosis, while another 30% were associated with cell-cycle pathways. The study also demonstrated the ability of ormeloxifene to inhibit cell proliferation by blocking cell cycle in the G0–G1 phase by upregulating p21 expression and inhibiting c-myc expression via ormeloxifene-induced c-myc promoter-binding protein (MBP-1) synthesis. The induction of apoptosis by ormeloxifene was associated with the ERK pathway and loss of mitochondrial membrane potential. Importantly, the results obtained from ormeloxifene treatment in the K562 cell line were translatable to mononuclear cells isolated from CML patients [56]. Ormeloxifene treatment of mononuclear cells isolated from patients showed apoptotic phenotypes, including nuclear condensation and membrane blebbing. These promising results suggest the development of ormeloxifene either alone or in combination with other chemotherapeutic agents is appropriate for the treatment of CML.
CONCLUSIONS, FUTURE PROSPECTS AND CHALLENGES
Cancer is one of the most widespread and deadly diseases in the world. It is a leading cause of death in both developing and developed countries, accounting for over 13% of deaths worldwide. Substantial resources and research has been dedicated towards understanding the causes of cancer and developing treatments/cures for this disease. However, very little progress has been made towards eradiation of this disease, since cancer is a complex, multi-component disease involving abnormal functioning of multiple genes or pathways within the cells, resulting in unregulated growth and proliferation [57]. The deregulation of multiple pathways that leads to cancer poses a major challenge for designing and developing new anti-cancer drugs with little or no side effects. The development of new drugs is a very expensive, involved and time consuming process, with few compounds passing the final stage for clinical use. Due to high mortality and morbidly in cancer patients worldwide, a smarter and more effective way to develop new anti-cancer agents might be the repurposing of established drugs such as ormeloxifene as anti-cancer agents. In fact, several examples exist wherein old clinically approved molecules have been successfully repurposed in new roles and functions. At present, many approved drugs, including metformin, are under investigation as anti-cancer agents. Most of the work on ormeloxifene as an anti-cancer agent has been reported in breast cancer. Recently, this drug has also been investigated for the inhibition of other cancers like chronic myeloid leukemia, as well as head and neck squamous cell carcinoma. Analysis of molecular mechanisms modulated by ormeloxifene suggests regulation of multiple signaling pathways in cancer cells (Fig. 2).
Although clinical studies have supported partial to complete remission of lesions in breast cancer patients after ormeloxifene treatment, comprehensive, multicenter clinical trials are required to approve worldwide utilization of this novel anti-cancer agent for cancer treatment. Also, research is required to reveal detailed molecular mechanisms and key cellular functions modulated by ormeloxifene in cancer cells. Some of the specific processes that need to be examined include the effect of ormeloxifene on tumor microenvironment and host immune system. In addition, investigations are required to delineate its effect on angiogenesis and epithelial mesenchymal transition (EMT) (important aspects of tumor development and metastasis). Furthermore, careful investigations on ormeloxifene in combination with other chemotherapy drug(s)/photochemical(s) are also required to determine useful synergistic combinations for treatment of refractory cancers. This information may help in developing a novel therapeutic regimen for the treatment of recalcitrant cancers.
Acknowledgments
The authors thank Cathy Christopherson and Jenna Klepatz for editorial assistance. This work was partially supported by grants from Governor’s Cancer 2010, the National Institutes of Health Research Project Grant Program (RO1) (CA142736), (UO1) (CA162106) and the Centers of Biomedical Research Excellence (COBRE: P20 RR024219).
ABBREVIATIONS
- SERM
Selective Estrogen Receptor Modulator
- ER
Estrogen Receptor
- DMBA
7,12-dimethylbenz-[a]anthracene
- HNSCC
Head and Neck Squamous Cell Carcinoma
- CML
Chronic Myeloid Leukemia
- MBP-1
c-Myc promoter-Binding Protein
- EMT
Epithelial Mesenchymal Transition
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
AUTHOR’S CONTRIBUTIONS
RG and VS designed and drafted the manuscript; MJ and SCC were involved in the critical revision of the manuscript and gave final approval of the version to be published. All authors read and approved the final manuscript.
References
- 1.Singh MM. Centchroman, a selective estrogen receptor modulator, as a contraceptive and for the management of hormone-related clinical disorders. Med Res Rev. 2001;21(4):302–347. doi: 10.1002/med.1011. [DOI] [PubMed] [Google Scholar]
- 2.Lal J. Clinical pharmacokinetics and interaction of centchroman-a mini review. Contraception. 2010;81(4):275–280. doi: 10.1016/j.contraception.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Singh MM, Kamboj VP. Fetal resorption in rats treated with an antiestrogen in relation to luteal phase nidatory estrogen secretion. Acta Endocrinol (Copenh) 1992;126(5):444–450. doi: 10.1530/acta.0.1260444. [DOI] [PubMed] [Google Scholar]
- 4.Misra NC, Nigam PK, Gupta R, Agarwal AK, Kamboj VP. Centchroman-a non-steroidal anti-cancer agent for advanced breast cancer: phase-II study. Int J Cancer. 1989;43(5):781–783. doi: 10.1002/ijc.2910430506. [DOI] [PubMed] [Google Scholar]
- 5.Nigam M, Singh N, Ranjan V, Zaidi D, Sharma R, Nigam D, Gupta DK, Sundaram S, Balapure AK. Centchroman mediated apoptosis involves cross-talk between extrinsic/intrinsic pathways and oxidative regulation. Life Sci. 2010;87(23–26):750–758. doi: 10.1016/j.lfs.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Nigam M, Ranjan V, Srivastava S, Sharma R, Balapure AK. Centchroman induces G0/G1 arrest and caspase-dependent apoptosis involving mitochondrial membrane depolarization in MCF-7 and MDA MB-231 human breast cancer cells. Life Sci. 2008;82(11–12):577–590. doi: 10.1016/j.lfs.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Shenoy BD, Udupa N, Kamath R, Devi PU. Evaluation of anti-tumor efficacy of injectable Centchroman in mice bearing Ehrlich ascites carcinoma. Indian J Physiol Pharmacol. 1999;43(2):259–262. [PubMed] [Google Scholar]
- 8.Srivastava VK, Gara RK, Bhatt ML, Sahu DP, Mishra DP. Centchroman inhibits proliferation of head and neck cancer cells through the modulation of PI3K/mTOR pathway. Biochem Biophys Res Commun. 2011;404(1):40–45. doi: 10.1016/j.bbrc.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Mukhopadhyay A, Ray S, Giri AK. Comparative antimutagenic effects of D- and L-centchroman and their comparison with tamoxifen in Salmonella assay. Mutat Res. 1999;445(1):1–8. doi: 10.1016/s1383-5718(99)00148-5. [DOI] [PubMed] [Google Scholar]
- 10.Roy S, Kumari GL, Madoiya K, Prakash V, Ray S. Induction of ovulation in the human with centchroman: a preliminary report. Fertil Steril. 1976;27(9):1108–1110. doi: 10.1016/s0015-0282(16)42083-2. [DOI] [PubMed] [Google Scholar]
- 11.Seth RK, Kole PL, Sarin JP. Studies on Centchroman, a new antifertility compound. Indian J Pharm Sci. 1983;45(1):14–16. [PubMed] [Google Scholar]
- 12.Ray S, Grover PK, Kamboj VP, Setty BS, Kar AB, Anand N. Antifertility agents. 12. Structure-activity relationship of 3,4-diphenylchromenes and -chromans. J Med Chem. 1976;19(2):276–279. doi: 10.1021/jm00224a014. [DOI] [PubMed] [Google Scholar]
- 13.Roy S, Datta JK. Nature of estrogenic and anti-estrogenic actions of centchroman on rat uterus. Contraception. 1976;13(5):597–604. doi: 10.1016/0010-7824(76)90015-9. [DOI] [PubMed] [Google Scholar]
- 14.Mehrotra PK, Karkun JN, Kar AB. Estrogenicity of some nonsteroidal compounds. Contraception. 1973;7(2):115–124. [Google Scholar]
- 15.Datta JK, Roy S. Effect of centchroman on morphological and biochemical changes induced by testosterone propionate in uterus of rats. Indian J Exp Biol. 1979;17(10):1061–1063. [PubMed] [Google Scholar]
- 16.Nair RK, Sheyte TA, Munshi SR. Progestational & antiprogestational effects of Centchroman in mouse & rabbit. Indian J Exp Biol. 1977;15(12):1157–1158. [PubMed] [Google Scholar]
- 17.Chandra H, Srimal RC, Kamboj VP, Dhawan BN, Gupta NN. Clinical pharmacology studies with Centchroman. Indian J Exp Biol. 1977;15(12):1170–1172. [PubMed] [Google Scholar]
- 18.Khurana M, Lal J, Singh MM, Paliwal JK, Kamboj VP, Gupta RC. Evaluation of interaction potential of certain concurrently administered drugs with pharmacological and pharmacokinetic profile of centchroman in rats. Contraception. 2002;66(1):47–56. doi: 10.1016/s0010-7824(02)00318-9. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava AK, Agnihotri A, Kamboj VP. Binding of centchroman--a nonsteroidal antifertility agent to human plasma proteins. Experientia. 1984;40(5):465–466. doi: 10.1007/BF01952388. [DOI] [PubMed] [Google Scholar]
- 20.Mishra N, Ratna S, Ray S, Roy S. Distribution and excretion pattern of 14C-labeled centchroman in the rhesus monkeys. Med Sci Res. 1992;20:259–260. [Google Scholar]
- 21.Ratna S, Mishra N, Ray S, Roy S. Centchroman: Tissue distribution and excretion profile in albino rats after oral and intravenous administration. J Basic Appl Biomed. 1994;2:31–34. [Google Scholar]
- 22.Paliwal JK, Gupta RC. Tissue distribution and pharmacokinetics of centchroman. A new nonsteroidal postcoital contraceptive agent and its 7-desmethyl metabolite in female rats after a single oral dose. Drug Metab Dispos. 1996;24(2):148–155. [PubMed] [Google Scholar]
- 23.Lal J, Asthana OP, Nityanand S, Gupta RC. Pharmacokinetics of centchroman in healthy female subjects after oral administration. Contraception. 1995;52(5):297–300. doi: 10.1016/0010-7824(95)00213-t. [DOI] [PubMed] [Google Scholar]
- 24.Paliwal JK, Grover PK, Asthana OP, Nityanand S, Gupta RC. Excretion of centchroman in breast milk. Br J Clin Pharmacol. 1994;38(5):485–486. doi: 10.1111/j.1365-2125.1994.tb04388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta RC, Nityanand S, Asthana OP, Lal J. Pharmacokinetics of Centchroman in Nursing Women and Passage into Breast Milk. Clin Drug Invest. 1996;11(5):305–309. [Google Scholar]
- 26.Lal J, Nityanand S, Asthana OP, Gupta RC. Multiple-dose pharmacokinetics of centchroman in female volunteers. Indian J Pharmacol. 1998;30:p120. [Google Scholar]
- 27.Singh MM, Chauhan SC, Trivedi RN, Maitra SC, Kamboj VP. Correlation of pinopod development on uterine luminal epithelial surface with hormonal events and endometrial sensitivity in rat. Eur J Endocrinol. 1996;135(1):107–117. doi: 10.1530/eje.0.1350107. [DOI] [PubMed] [Google Scholar]
- 28.Chauhan SC, Singh MM, Maitra SC, Kamboj VP. Inhibition of progesterone-induced development of giant mitochondria in uterine glandular epithelial cells by an antiestrogen in rat. Contraception. 1996;54(4):259–264. doi: 10.1016/s0010-7824(96)00197-7. [DOI] [PubMed] [Google Scholar]
- 29.Arbatti NJ, Sheth AR, Vaidya RA. Effect of L-dopa on Centchroman induced prolactin levels in female rats. Indian J Exp Biol. 1977;15(12):1193–1194. [PubMed] [Google Scholar]
- 30.Arbatti NJ, Sheth AR, Vaidya RA. Mode of action of Centchroman on hypothalamo-pituitary axis in male rats. Indian J Exp Biol. 1977;15(12):1194–1195. [PubMed] [Google Scholar]
- 31.Saha Roy S, Vadlamudi RK. Role of estrogen receptor signaling in breast cancer metastasis. Int J Breast Cancer. 2012;2012:Article ID 654698. doi: 10.1155/2012/654698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim E, Metzger-Filho O, Winer EP. The natural history of hormone receptor-positive breast cancer. Oncology (Williston Park) 2012;26(8):688–694. [PubMed] [Google Scholar]
- 33.Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011;11(8):597–608. doi: 10.1038/nrc3093. [DOI] [PubMed] [Google Scholar]
- 34.Dwivedi A, Basu R, Chowdhury SR, Goyal N. Modulation of estrogen action during preimplantation period and in immature estradiol-primed rat uterus by anti-implantation agent, ormeloxifene. Contraception. 2005;71(6):458–464. doi: 10.1016/j.contraception.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 35.O’Connor PM, Jackman J, Bae I, Myers TG, Fan S, Mutoh M, Scudiero DA, Monks A, Sausville EA, Weinstein JN, Friend S, Fornace AJ, Jr, Kohn KW. Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res. 1997;57(19):4285–4300. [PubMed] [Google Scholar]
- 36.Singh N, Zaidi D, Shyam H, Sharma R, Balapure AK. Polyphenols sensitization potentiates susceptibility of MCF-7 and MDA MB-231 cells to Centchroman. PLoS One. 2012;7(6):e37736. doi: 10.1371/journal.pone.0037736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yallapu MM, Maher DM, Sundram V, Bell MC, Jaggi M, Chauhan SC. Curcumin induces chemo/radio-sensitization in ovarian cancer cells and curcumin nanoparticles inhibit ovarian cancer cell growth. J Ovarian Res. 2010;3(11) doi: 10.1186/1757-2215-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishra R, Tiwari A, Bhadauria S, Mishra J, Murthy PK, Murthy PS. Therapeutic effect of centchroman alone and in combination with glycine soya on 7,12-dimethylbenz[alpha]anthracene-induced breast tumor in rat. Food Chem Toxicol. 2010;48(6):1587–1591. doi: 10.1016/j.fct.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 39.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 40.Ho SM, Leung YK, Chung I. Estrogens and Antiestrogens as Etiological Factors and Therapeutics for Prostate Cancer. Ann N Y Acad Sci. 2006;1089(1):177–193. doi: 10.1196/annals.1386.005. [DOI] [PubMed] [Google Scholar]
- 41.Lissoni P, Vigano P, Vaghi M, Frontini L, Giuberti C, Manganini V, Casu M, Brivio F, Niespolo R, Strada G. A phase II study of tamoxifen in hormone-resistant metastatic prostate cancer: possible relation with prolactin secretion. Anticancer Res. 2005;25(5):3597–3599. [PubMed] [Google Scholar]
- 42.Clarke BL, Khosla S. Modulators of androgen and estrogen receptor activity. Crit Rev Eukaryot Gene Expr. 2010;20(4):275–294. doi: 10.1615/critreveukargeneexpr.v20.i4.10. [DOI] [PubMed] [Google Scholar]
- 43.Mimeault M, Venkatraman G, Johansson SL, Moore E, Henichart JP, Depreux P, Lin MF, Batra SK. Novel combination therapy against metastatic and androgen-independent prostate cancer by using gefitinib, tamoxifen and etoposide. Int J Cancer. 2007;120(1):160–169. doi: 10.1002/ijc.22268. [DOI] [PubMed] [Google Scholar]
- 44.Rossi V, Bellastella G, De Rosa C, Abbondanza C, Visconti D, Maione L, Chieffi P, Della Ragione F, Prezioso D, De Bellis A, Bellastella A, Sinisi AA. Raloxifene induces cell death and inhibits proliferation through multiple signaling pathways in prostate cancer cells expressing different levels of estrogen receptor alpha and beta. J Cell Physiol. 2011;226(5):1334–1339. doi: 10.1002/jcp.22461. [DOI] [PubMed] [Google Scholar]
- 45.Matta A, Ralhan R. Overview of current and future biologically based targeted therapies in head and neck squamous cell carcinoma. Head Neck Oncol. 2009;1(6) doi: 10.1186/1758-3284-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuller DE, Abou-Issa H, Parrish R. Estrogen and progesterone receptors in head and neck cancer. Arch Otolaryngol. 1984;110(11):725–727. doi: 10.1001/archotol.1984.00800370027006. [DOI] [PubMed] [Google Scholar]
- 47.Shatalova EG, Klein-Szanto AJP, Devarajan K, Cukierman E, Clapper ML. Estrogen and Cytochrome P450 1B1 Contribute to Both Early- and Late-Stage Head and Neck Carcinogenesis. Cancer Prev Res. 2011;4(1):107–115. doi: 10.1158/1940-6207.CAPR-10-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Egloff AM, Rothstein ME, Seethala R, Siegfried JM, Grandis JR, Stabile LP. Cross-Talk between Estrogen Receptor and Epidermal Growth Factor Receptor in Head and Neck Squamous Cell Carcinoma. Clin Cancer Res. 2009;15(21):6529–6540. doi: 10.1158/1078-0432.CCR-09-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alonso L, Gallego E, Gonzalez FJ, Sanchez-Munoz A, Torres E, Pajares BI, Leeflang S, Baha C. Gonadotropin and steroid receptors as prognostic factors in advanced ovarian cancer: a retrospective study. Clin Transl Oncol. 2009;11(11):748–752. doi: 10.1007/s12094-009-0437-4. [DOI] [PubMed] [Google Scholar]
- 50.Lavie O, Longacre T, Segev Y, Husain A. Ovarian carcinosarcomas associated with prolonged use of tamoxifen: case reports. Int J Gynecol Cancer. 2009;19(9):1521–1523. doi: 10.1111/IGC.0b013e3181a83fbf. [DOI] [PubMed] [Google Scholar]
- 51.Vermorken JB, Hoekman K. Chemotherapy for gynecologic malignancies. Curr Opin Oncol. 1995;7(5):457–465. doi: 10.1097/00001622-199509000-00012. [DOI] [PubMed] [Google Scholar]
- 52.Bendrik C, Karlsson L, Dabrosin C. Increased endostatin generation and decreased angiogenesis via MMP-9 by tamoxifen in hormone dependent ovarian cancer. Cancer Lett. 2010;292(1):32–40. doi: 10.1016/j.canlet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 53.Mullen P, Cameron DA, Hasmann M, Smyth JF, Langdon SP. Sensitivity to pertuzumab (2C4) in ovarian cancer models: cross-talk with estrogen receptor signaling. Mol Cancer Ther. 2007;6(1):93–100. doi: 10.1158/1535-7163.MCT-06-0401. [DOI] [PubMed] [Google Scholar]
- 54.Faratian D, Zweemer AJ, Nagumo Y, Sims AH, Muir M, Dodds M, Mullen P, Um I, Kay C, Hasmann M, Harrison DJ, Langdon SP. Trastuzumab and pertuzumab produce changes in morphology and estrogen receptor signaling in ovarian cancer xenografts revealing new treatment strategies. Clin Cancer Res. 2011;17(13):4451–4461. doi: 10.1158/1078-0432.CCR-10-2461. [DOI] [PubMed] [Google Scholar]
- 55.Devalapally H, Duan Z, Seiden MV, Amiji MM. Paclitaxel and ceramide co-administration in biodegradable polymeric nanoparticulate delivery system to overcome drug resistance in ovarian cancer. Int J Cancer. 2007;121(8):1830–1838. doi: 10.1002/ijc.22886. [DOI] [PubMed] [Google Scholar]
- 56.Pal P, Kanaujiya JK, Lochab S, Tripathi SB, Bhatt ML, Singh PK, Sanyal S, Trivedi AK. 2-D gel electrophoresis-based proteomic analysis reveals that ormeloxifen induces G0–G1 growth arrest and ERK-mediated apoptosis in chronic myeloid leukemia cells K562. Proteomics. 2011;11(8):1517–1529. doi: 10.1002/pmic.201000720. [DOI] [PubMed] [Google Scholar]
- 57.Cao Y, DePinho RA, Ernst M, Vousden K. Cancer research: past, present and future. Nat Rev Cancer. 2011;11(10):749–754. doi: 10.1038/nrc3138. [DOI] [PubMed] [Google Scholar]