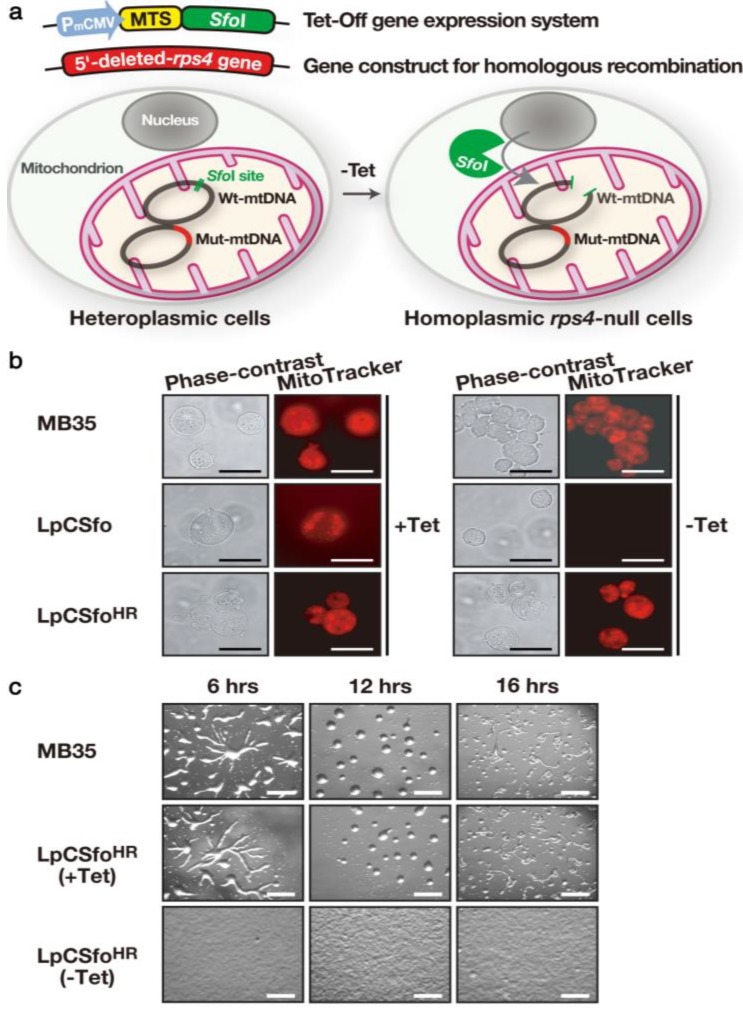
Figure 3.
Strategy for creating rps4-null cells and their phenotypes. (a) As a starting material, LpCSfo cells in which pCoxIV (MTS)-SfoI is expressed under the tetracycline-minus (−Tet) condition, were prepared. Subsequently, the mutant rps4 gene (Mut-mtDNA for homologous recombination), in which the upstream SfoI-site and a 5'-half of rps4 coding region were deleted, was introduced into LpCSfo cells to obtain heteroplasmic transformants (LpCSfoHR cells) with mitochondria consisting of the Mut-mtDNA and wild-type mtDNA (Wt-mtDNA). Coupled with removal of Tet from growth medium, the fusion protein MTS-SfoI synthesized in the cytoplasm is exclusively transferred into mitochondria of LpCSfoHR cells and selectively digests Wt-mtDNA but not Mut-mtDNA. Since the digested Wt-mtDNA is not duplicated, the Mut-mtDNA becomes dominant during the course of growth under the −Tet condition, thus eventually giving rps4-null cells. (b) These cells were grown in growth medium with (+Tet) or without (−Tet) teteracyclin. Membrane potential of mitochondria was visualized by staining of cells with MitoTracker Orange. As was expected, the staining of mitochondria was almost completely vanished in LpCSfo cells grown without Tet, because their mtDNA with an intact SfoI site would be cleaved by SfoI eventually to become a ρ0 state devoid of mitochondrial DNA. Bars, 200 nm. (c) Development of starved MB35 cells and LpCSfoHR cells on agar. MB35 cells and LpCSfoHR cells grown with (+Tet) or without (−Tet) tetracycline were washed twice in BSS and plated on 1.5% non-nutrient agar at a density of 5 × 106 cells/cm2. This was followed by incubation for the indicated time at 22 °C. Bars, 0.5 mm. (Basically from Chida et al. [22]).