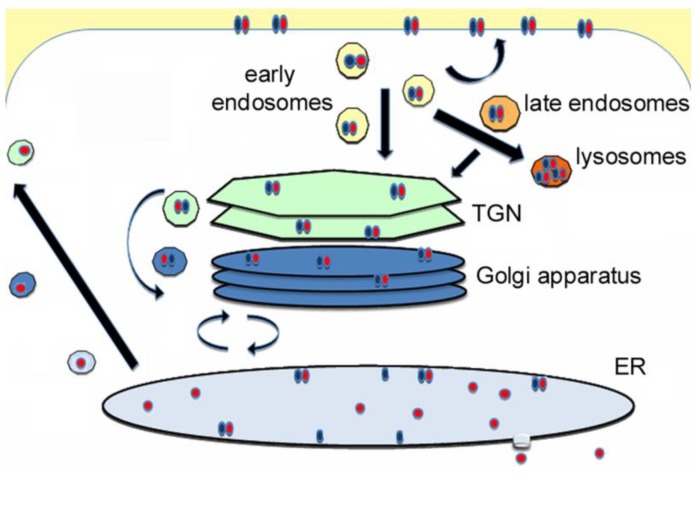
Figure 1.
Intracellular toxin trafficking. The general trafficking and translocation itinerary for AB-type, endoplasmic reticulum (ER)-translocating toxins is shown. These toxins bind to distinct surface receptors and are internalized by a variety of endocytic mechanisms. The internalized toxin is recycled to the plasma membrane, directed to the lysosomes for degradation, or delivered to the trans-Golgi network (TGN) en route to the ER translocation site. Vesicle-mediated transport to the TGN can originate from the early or late endosomes, depending on which toxin is present. Likewise, multiple retrograde transport pathways can deliver the toxin from the TGN to the ER. The toxin may cycle between the Golgi and ER until the catalytic subunit dissociates from the rest of the toxin and shifts to an unfolded conformation which triggers its export to the cytosol in a process involving the quality control system of ER-associated degradation. Some of the free, ER-localized A chain escapes ER-associated degradation (ERAD) and is secreted back into the medium via Golgi and TGN intermediates. In most cell types, trafficking from the cell surface to the ER is very inefficient: the majority of internalized toxin is routed to the lysosomes, and only around 10% of surface-bound toxin reaches the ER [9,10,11,12,13,14,15,16]. Thus, ectopic expression of an ER-localized A chain via transfected cultured cells, transformed yeast, or microsomal transcription/translation systems is often used for toxin translocation studies.