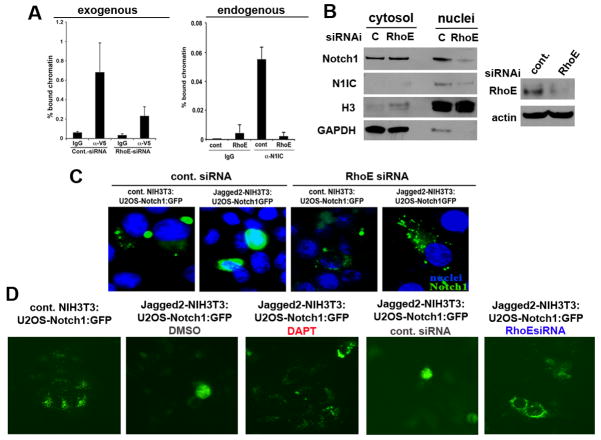
Figure 7. RhoE promotes N1IC transcriptional activity and importin β1-mediated nuclear import.
A, RhoE knock-down inhibits Notch1-induced CSL-transcription in normal keratinocytes. Human primary keratinocytes under growing conditions were transfected with control siRNA, or RhoE siRNA followed by infection with an adenoviral construct encoding the activated form of Notch1 with a V5 C-terminal tag. 24 hours later cells were processed for ChIP with V5 specific antibodies or non-immune IgG control followed by PCR amplification of the CSL/Notch binding site-containing region of the human HES1 promoter (left panel). Differentiated primary HKC were processed for ChIP as in Figure 3 using endogenous N1IC for immunoprecipitation (right panel). The results from the ChIP assay were quantified by real time PCR analysis. Error bars represent Standard Deviations, calculated based on three independent measurements. B, RhoE inhibition prevents accumulation of activated Notch1 in the nucleus. Primary HKC transfected with RhoE siRNA were induced to differentiate by suspension culture, followed by nuclear and cytoplasmic fractionation and immunoblot analysis for the full length and activated Notch1 proteins using H3 and GAPDH as loading controls for the nuclear and cytosolic fractions, respectively. RhoE knockdown was assessed by Western blot analysis as shown in the right panel. C, U2OS cells stably expressing the full length Notch1-GFP (the GFP tag is at the intracellular end of the protein) transfected with control or RhoE siRNA and seeded over NIH3T3 cells expressing control empty vector or full length Jagged2 were analyzed for ligand mediated translocation of cleaved Notch1-GFP to the nucleus 24hrs later by fluorescent microscopy. D, Same co-culture system as in C but imaging was performed on live cells and the effects of RhoE siRNA were compared to DAPT treatment.