Abstract
Rationale
Both β-adrenergic (β-AR) and Gq-coupled agonist (GqR) driven signaling play key roles in the events leading up to and during cardiac dysfunction. How these stimuli interact at the level of protein kinase D (PKD), a nodal point in cardiac hypertrophic signaling, remains unclear.
Objective
To assess the spatiotemporal dynamics of PKD activation in response to β-AR signaling alone and upon co-activation with GqR agonists. This will test our hypothesis that compartmentalized PKD signaling reconciles disparate findings of protein kinase A (PKA) facilitation and inhibition of PKD activation.
Methods and Results
We report on the spatial and temporal profiles of PKD activation using GFP-tagged PKD (wildtype or mutant S427E) and targeted FRET based biosensors (DKARs) in adult cardiomyocytes. We find that β-AR/PKA signaling drives local nuclear activation of PKD, without preceding sarcolemmal translocation. We also discover pronounced interference of β-AR/cAMP/PKA signaling on GqR-induced translocation and activation of PKD throughout the cardiomyocyte. We attribute these effects to direct, PKA-dependent phosphorylation of PKD-S427. We also show that phosphomimetic substitution of S427 likewise impedes GqR-induced PKD translocation and activation. In neonatal myocytes, S427E inhibits GqR-evoked cell growth and expression of hypertrophic markers. Lastly, we show altered S427 phosphorylation in TAC-induced hypertrophy.
Conclusions
β-AR signaling triggers local nuclear signaling and inhibits GqR-mediated PKD activation by preventing its intracellular translocation. PKA-dependent phosphorylation of PKD S427 fine-tunes the PKD responsiveness to GqR-agonists, serving as a key integration point for β-adrenergic and Gq-coupled stimuli.
Keywords: Protein kinase D, β-adrenergic signaling, protein kinase A, cardiac myocytes, adrenergic receptor, cardiac, G proteins
INTRODUCTION
Cardiac remodeling occurs after various types of insults to the heart including inflammation and pressure and volume overload.1, 2 Associated molecular, cellular and interstitial changes, such as re-expression of fetal genes, myocyte hypertrophy, cell death and fibrosis, eventually become maladaptive leading to cardiac dysfunction and failure. Therapeutic strategies therefore often target the signaling pathways that underlie the cardiac remodeling processes. In recent years protein kinase D (PKD) has emerged as a key signaling nodal point affecting excitation-contraction coupling (via myofilament binding protein C, cardiac Troponin I and L-type Ca2+ channels),3–6 gene expression (via histone deacetylases (HDACs)7, 8 and cAMP-response element binding protein (CREB)9), cell survival10–12 and energy substrate utilization.13–15
In vivo, cardiac-specific expression of constitutively active PKD1 caused pronounced dilated cardiomyopathy,7 while cardiac-specific PKD1 knockout mice proved remarkably resistant to cardiac hypertrophy and fibrosis in response to pressure overload or chronic administration of adrenergic or angiotensin receptor agonists.16 Conversely PKD inhibition decreased the tolerance to ischemia/reperfusion injury.17 PKD expression and activity are also increased in failing rat,7 rabbit and human myocardium.18 These findings indicate the importance of PKD in modulating cardiac pathophysiology and its potential as a therapeutic target for cardiovascular disease.
Three highly homologous PKD isoforms have been identified (PKD1-3, with PKD1 predominant in the heart).2, 19 PKDs possess substrate specificity similar to CaMK and structural features reminiscent of PKCs. Indeed PKD’s modular structure consists of a C-terminal catalytic domain and an N-terminal region with autoinhibitory, regulatory domains such as the diacylglycerol (DAG) binding domain and the pleckstrin homology domain.20, 21 Several regulatory mechanisms (e.g.22 for comprehensive review) can modulate PKD1 activity including autoinhibition, phosphorylation, proteolytic degradation, subcellular localization and protein interactions resulting in complex, context-specific PKD signaling mechanisms.
The typical signaling cascade to PKD activation involves GPCR-dependent activation of phospholipase C and production of DAGs. The latter stimulate PKD directly and indirectly via coincident activation of PKCs while recruiting PKD near its upstream kinase. The activation is largely dependent on phosphorylation of the activation loop residues (S744/748 in murine numbering) by both PKC-dependent and -independent mechanisms.23–26 Despite this general consensus on global PKD activation for GPCR agonists, our group27, 28 recently identified dramatic spatiotemporal differences in PKD1 activation for two Gq-coupled agonists, phenylephrine (PE) and endothelin-1 (ET1) in adult cardiomyocytes. While both agonists triggered comparable global PKD1 activation, PE induced fleeting sarcolemmal PKD1 translocation and activation followed by nuclear translocation and ET1 prompted persistent sarcolemmal recruitment and activity. The ability to dynamically alter the location and signal generation of PKD1 is clearly an integral part of achieving PKD signal specificity yet this highly contextual and microdomain aspect of PKD signaling remains poorly understood.
Surprisingly, little is known of the effect of β-AR/PKA signaling on PKD function and activation in cardiomyocytes and the few existing reports are conflicting. Initial reports from the Olson group saw no effect of β-AR or PKA stimulation on PKD phosphorylation or activity.7 Carnegie and colleagues later found that AKAP-Lbc functions as a scaffold for PKA and PKC, facilitating PKD activation and the transduction of hypertrophic responses (via HDAC5 and Mef2).29–31 Others, examining global PKD activity, report a counter modulatory role for PKA and PKC on PKD activation in adult myocytes.32–34 That is an inhibitory role for PKA.
In the present study, we resolve these conflicting findings by examining local PKD translocation and activation in response to PKA signaling alone and the crosstalk between these two signaling kinases in adult cardiac myocytes. We find that β–AR/PKA signaling drives nuclear activation of PKD without requiring a sarcolemmal recruitment phase. β-AR/PKA signaling also prevents GqR-induced translocation, activation and function of PKD and we show that these effects are mediated by PKA-dependent phosphorylation of PKD at S427. Moreover, initial observations in a TAC-induced hypertrophy model indicate altered phosphorylation of this S427 site. Our study provides further evidence of compartmentalized PKD signaling and indicates how S427 could serve as a critical control point for modulation of the spatiotemporal dynamics of PKD activity and function during the pathogenesis of cardiac disease.
METHODS
Cardiomyocyte isolation and culture
All animal and biohazard procedures were conducted in accordance with NIH guidelines for animal research and with institutional approval. Biohazard procedures were performed in accordance with a UC Davis approved Biological Use Authorization. Adult myocytes were isolated and cultured as previously described27, 35. Neonatal rat cardiomyocytes (NCM) were cultured as instructed. GFP-PKD-WT and -S427E expression was via adenoviral infection. Prior to experiments myocytes were serum-deprived for 24 hours by switching to DMEM with Nutridoma-SP.
Confocal, FRET and Total Internal Reflection Fluorescence (TIRF) measurements
All confocal and TIRF experiments were performed as previously reported.27 Myocytes were exposed to inhibitors for 15–20 minutes prior to and following addition of agonists. FRET was measured27 using both a ratiometric and an acceptor photobleach approach. Background corrected fluorescence intensities at specific cellular locations were reported as a ratio of FCFP/FYFP (normalized to initial ratio).
Immunoblotting
Cell lysates and subcellular fractions were obtained as previously described27, 36 and probed with: from Cell Signaling PKD1, PKDpS916, phospho-PKA-Substrate (RRXS*/T*), phospho-Akt Substrate (RXXS*/T*), phospho-PKD-substrate and histone 3; from Abcam GAPDH, PKDpS916, PKA and a custom PKDpS427 antibody; from Santa Cruz Biotechnology GFP and from Millipore NKAα1. Immunoreactive band intensities were quantified using ImageJ.
Immunoprecipitation (IP)
Immunoprecipitation was performed as before37 using anti-GFP, PKD or PKARII antibody at 4°C overnight followed by incubation with proteinA/G-coupled magnetic beads for 2 hours before resuspending in sample buffer.
Sequence alignment
Sequence alignment and analysis were performed using Geneious version 6.0.4 by Biomatters. (http://www.geneious.com/).
Cellular hypertrophy
NCMs were treated as indicated before subjecting to standard immunocytochemistry and RT-PCR of hypertrophic markers. Fixed NCM were stained for α-actinin (Sigma, 1:1000). Cell size measurements were made using Image J. Total RNA was isolated using TRIZOL Reagent and NucleoSpin® RNA II, followed by quantification using Nanodrop and then Quant-iT™ RiboGreen. cDNA was generated from RNA with RNA-to-cDNA-EcoDry-Premix and then amplified with GoTaq® qPCRMasterMix. Custom primers (Illumina) were used for detection of each mRNA:Control genes: Hmbs, Rpl13a, Hprt1, Myh6, Myh7, Acta1, Nppb and Nppa. The threshold crossing value was noted for each transcript and normalized to internal control. The relative quantification of each was performed using the comparative Ct method.
Transverse aortic constriction (TAC) surgery
All procedures were essentially as described38. TAC was performed on 8-week-old male mice around a 27-gauge needle. SHAM-operated mice were subjected to identical interventions except for aortic constriction. Cardiac dysfunction was measured 3 weeks later via echocardiography. The mice were sacrificed and hearts were harvested. For cardiac homogenates, the heart was powdered and resuspended in ice-cold buffer containing (in mmol/L): 10 Na2HPO4, 150 NaCl, 5 EGTA, 5 EDTA, 5 NaF, 1 orthovanadate with 1% TritonX-100, 1% Na Deoxycholate, and protease and phosphatase inhibitors. After homogenization with polytron at 5000rpm for 20sec and 5 strokes of a Dounce homogenizer, samples were centrifuged at 5,000g. Supernatants were flash-frozen.
Statistical analysis
Pooled data are expressed as mean±SEM. Statistical discriminations were performed with Student’s t-test and 2-way-ANOVA with p<0.05 considered significant.
Expanded methods can be found in the online supplement.
RESULTS
β-AR/PKA drives non-canonical PKD translocation
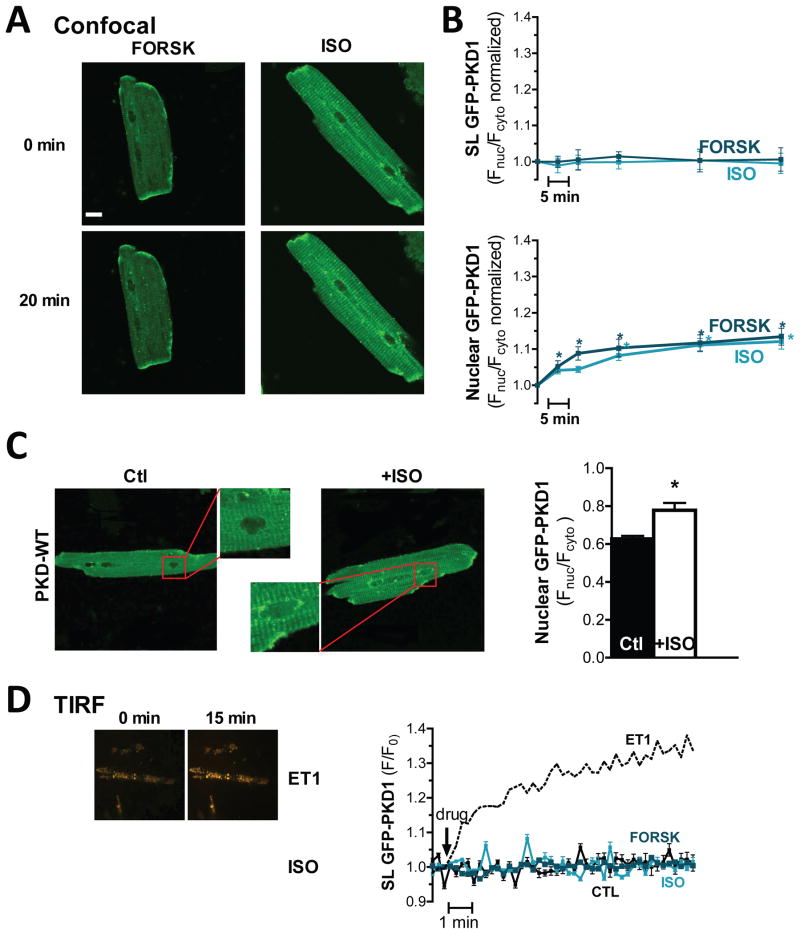
Initial experiments focused on establishing whether β-AR or PKA activation alone affects PKD signaling and localization. Adult cardiomyocytes expressing GFP-PKD1 (following adenovirus infection), were monitored via confocal microscopy following 100nM isoproterenol (ISO) or 10μM forskolin (Forsk) exposure (Fig 1A). Quantification of the signal at the sarcolemma vs. that in the cytosol (FSL/Fcyto) showed that neither ISO nor Forsk induced sarcolemmal recruitment of PKD (Fig 1B). This is in contrast to the pronounced sarcolemmal recruitment of PKD we previously observed in response to the GqR agonists PE and ET1.28 Notably, even without a preceding sarcolemmal translocation phase, both ISO and Forsk triggered nuclear import of PKD (after 60 min Fnuc/Fcyto was increased by 12±2% and 13±2%, respectively; Fnuc contains both intranuclear and nuclear membrane fluorescence intensities). This nuclear import was also readily detectable in population studies of PKD distribution in response to ISO (i.e. unpaired measurements of Fnuc/Fcyto, Fig 1C).
Figure 1. β-AR/PKA activation triggers non-classical PKD translocation.
(A) Confocal images of rabbit ventricular myocytes expressing GFP-tagged PKD1 before and after 20min of 10μM Forsk treatment (left) or 100nM ISO (right). (B) PKD-GFP localization was analyzed as FSL/Fcyto and FNuc/Fcyto for sarcolemmal recruitment and nuclear import respectively (n>13 myocytes, *P<0.001). (C) Population analysis of ISO-triggered nuclear import of GFP-PKD1: confocal images (left) and quantification as FNuc/Fcyto (right). (D) TIRF analysis of agonist-dependent sarcolemmal recruitment of GFP-PKD1. Representative TIRF images in response to ET1 or ISO are on the left. Quantification of GFP-PKD1 TIRF signals is shown on the right (n>10 myocytes).
To more selectively measure sarcolemmal recruitment of PKD, we also used total internal reflection fluorescence (TIRF) microscopy to assess fluorescence only within ~100nm of the glass coverslip-solution interface (Fig 1D). As in the confocal experiments, neither the β-AR agonist ISO nor direct activation of adenylyl cyclase by Forsk induced significant sarcolemmal recruitment of PKD. This suggests that β-AR/PKA activation triggers a translocation pattern that deviates from the classical PKD activation pattern of a membrane recruitment phase followed by translocation to subcellular targets such as the nucleus.
cAMP-dependent signaling selectively activates nuclear PKD
Using genetically encoded FRET-based D-kinase activity reporter (DKAR), we previously showed that the triggered PKD translocation parallels the evoked local kinase activity.28 Because of the possibility that PKD may be activated in the absence of prior sarcolemmal translocation, we used targeted DKARs (to nucleus, cytosol or sarcolemma) to selectively monitor the time course and magnitude of PKD activation in response to ISO or Forsk. Phosphorylation of the PKD-specific substrate sequence in this biosensor, results in a decrease in FRET (i.e. an increase in CFP/YFP ratio).39 Although the DKAR dynamic range is small (~10%), they are able to detect spatiotemporal differences in PKD activation.28 We found that exposure of adult cardiac myocytes to ISO or Forsk failed to elicit any PKD activation at the sarcolemma or cytosol, measured with DKAR-MyrP and DKAR-NES respectively (Fig 2A). However both ISO and Forsk activated nuclear PKD (Fig 2A). The effect was more potent for Forsk, reflecting the more robust PKA activation. We confirmed these results using the acceptor photobleach method (not shown). These observations support the notion that PKA activation induces nuclear translocation and activation of PKD without a preceding sarcolemmal recruitment and activation phase.
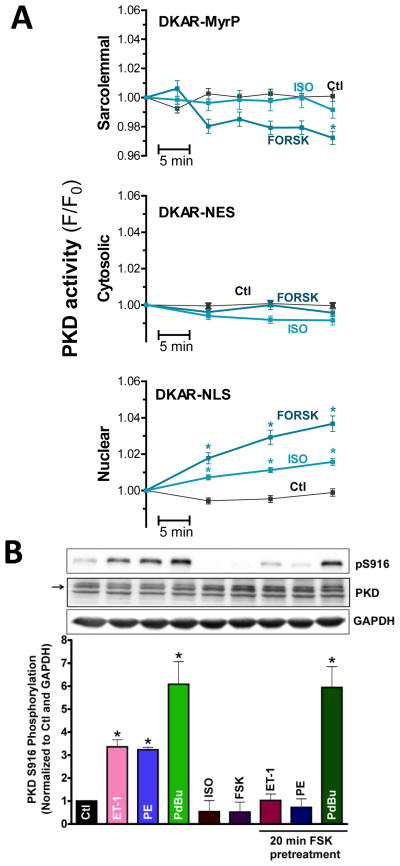
Figure 2. β-AR/PKA trigger nuclear activation of PKD.
(A) PKD activity measurements with targeted DKAR constructs in sarcolemmal, cytosol and nuclear compartments (n>30 myocytes, *P<0.01). (B) Representative western blot and quantification of S916 phosphorylation (~global PKD activity) in response to agonist treatment. GAPDH and PKD signals serve as loading controls.
We also used immunoblotting (Fig 2B) to assess global effects on PKD activity in response to β-AR or PKA. For these in vitro measurements, autophosphorylation at S916 was used as readout of PKD activity. Consistent with our previously published findings, myocyte exposure (for 20 min) to PDBu (a phorbolester), PE or ET1 significantly increased PKD S916 autophosphorylation compared to untreated myocytes. In contrast, ISO or Forsk treatment did not alter baseline S916 phosphorylation. The lack of an effect on global PKD autophosporylation status may be due to the relatively minor contribution of nuclear PKD to that of the whole cell (estimated to be <3% of total PKD based on subcellular compartment estimates of total cell volume). So, fractionated mouse hearts (± ISO) were analyzed for PKD activation (via pS916 signals) and phosphorylation of PKD targets (using a PKD consensus motif antibody). While S916 and PKD target phosphorylation signals were slightly decreased in the cytosolic and sarcolemmal fractions, they were increased in the nuclear compartment (Supplemental Fig I). These biochemical observations confirm β-AR signaling stimulates local, nuclear PKD signaling.
Having established the effect on PKD of β-AR/PKA signaling alone, we set out to determine how this pathway affects signaling through GqR/PKD pathways (Fig 2B). We found that pre-activation of PKA by Forsk prevented the increase in S916 phosphorylation induced by ET1 and PE. In contrast, Forsk pretreatment failed to prevent S916 autophosphorylation in response to PDBu. These results indicate that PKA dependent signaling suppresses global PKD activation in cardiomyocytes in response to GqR agonists, at least as sensed by S916 phosphorylation. Potent stimuli, like the DAG analogue PDBu, are still able to overcome this inhibition.
β-AR/PKA negatively modulates Gq-driven PKD translocation
Clearly there are subcellular, compartmentalized responses to PKA- and GqR-dependent activation of PKD. To assess whether microdomain signaling plays a role in this PKA-dependent modulation of Gq-coupled PKD activation, we examined PKD-GFP translocation in adult cardiomyocytes. Consistent with prior observations confocal imaging revealed pronounced sarcolemmal translocation in response to PE and ET1 (stronger and sustained for ET1), followed by nuclear translocation, which is more rapid and prominent for PE. ISO pretreatment prevented both the ET1- and PE-induced PKD translocation to the sarcolemma (Fig 3B, left). FSL/Fcyto at 60 min remained at baseline for ISO-pretreated myocytes vs. an increase of 9±1% and 31±4% for PE and ET1 alone. The GqR-agonist mediated nuclear import of PKD was also blocked by pre-incubation with ISO (Fig 3B, right). The PE-triggered increase in Fnuc/Fcyto ratio after 60 min was only 1±2% in ISO-pretreated myocytes (vs. 18±2% for PE alone). Likewise, the increase in Fnuc/Fcyto ratio was only 2±1% after 60 min of ET1 in ISO-pretreated cells (from 13±3%). Of note, the ISO induced nuclear import had already occurred within the 15 min pretreatment period (an ~10% increase). This higher, new baseline (reset to 1) was used to estimate the GqR.
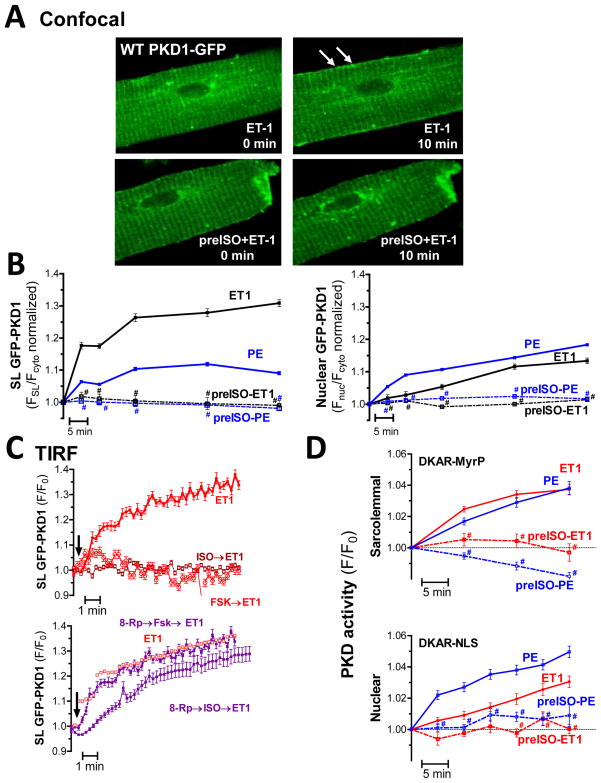
Figure 3. β-AR/PKA signaling prevents GqR-induced PKD translocation and activation.
(A) Confocal images of GFP-PKD1 translocation in response to ET1 alone (top) or following ISO pretreatment (bottom). (B) Analysis of GFP-PKD1 localization shows ISO pretreatment blocks both PE-and ET1-induced sarcolemmal recruitment (left) and nuclear import (right) (n=11–30, * P<0.05, SL recruitment for PE and ET1 was also significantly different). (C) TIRF measurements of PKD sarcolemmal recruitment in response to GqR-agonists ± pretreatment with ISO or Forsk. Bottom panels show PKA inhibition with Rp-8-Br-cAMP prevents the ISO and Forsk effect. (n>5 myocytes) (D) PKD activity measurements with targeted DKAR constructs in response to GqR agonists ± pretreatment with ISO (n> 30 myocytes, *P<0.001).
To corroborate our confocal findings, we used TIRF microscopy to dissect the ISO-induced signaling cascade leading to alterations in PE/ET1-induced PKD translocation. Our TIRF measurements confirmed that β–AR activation with ISO (15min pretreatment) prevents ET1 induced sarcolemmal recruitment of PKD in adult myocytes (Fig 3C, top). Elevating cAMP levels with Forsk to more directly activate PKA likewise blocked the ET1 effects. Consistent with these observations, pre-incubation with 8-Rp-Br-cAMP, a cell-permeable cAMP analogue, which very specifically blocks PKA activation, prevented the ISO-and Forsk-dependent inhibitory effects on ET1-driven PKD translocation (Fig 3C). Similar results were obtained for PE (Supplemental Fig II), even though the PE-induced SL retention of PKD is lower than for ET1. Taken together these data indicate that signaling through a β–AR/cAMP/PKA pathway negatively regulates GqR-agonist evoked PKD translocation.
β-AR signaling negatively modulates local PKD activity
Targeted DKARs (Fig 3D) were used in parallel studies to test how GqR-agonists affect the spatiotemporal dynamics of PKD activation after β-AR stimulation. While ET1 and PE triggered comparable sarcolemmal PKD activity, ISO pretreatment blocked ET1-or PE-induced PKD activation at the sarcolemma (top). PE and ET1 also triggered nuclear PKD activation, though to a much greater extent for PE. ISO pretreatment again effectively reduced PKD activation in the nuclear compartment in response to PE or ET1 (bottom). Collectively, our data show that β-AR/cAMP/PKA signaling profoundly perturbs GqR-agonist induced PKD activation and translocation in adult cardiomyocytes. Parallel subcellular fractionation experiments and probing for activated PKD (pS916) support these findings (Supplemental Fig III). As shown for the sarcolemmal compartment, PE- and ET1-induced increase in signal is prevented by ISO pretreatment.
PKA-dependent phosphorylation of PKD
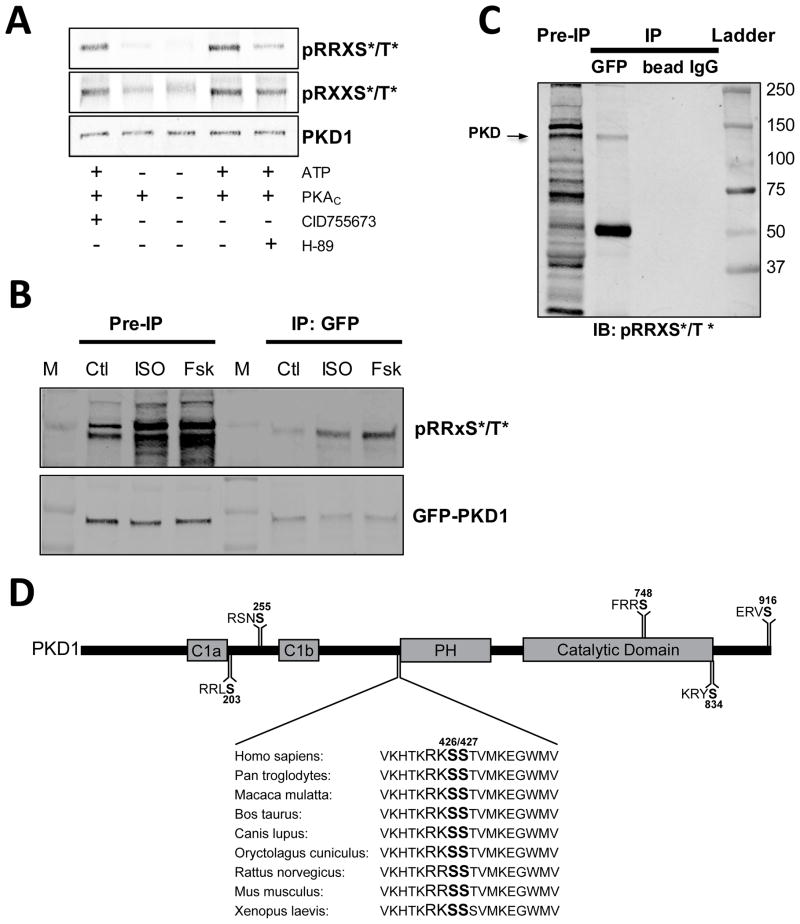
Thus far, it was unclear whether the disruption of Gq-induced PKD signaling is mediated via direct PKA phosphorylation of PKD or other PKA-mediated effects. So we used two separate PKA-consensus-motif antibodies (pRRXS*/T* and pRXXS*/T*) to test whether PKA can directly phosphorylate PKD. Western blots of purified PKD1 phosphorylated in vitro with PKA show increased immunoreactivity with both PKA-consensus-motif antibodies (Fig 4A). H-89 inhibition of PKA strongly reduced the signals, whereas PKD inhibition with CID755673 had little effect. Thus PKD is a PKA substrate. Moreover, PKD can be co-immunoprecipitated with the PKA regulatory subunit (Supplemental Fig IV) in myocyte lysates. So we tested whether PKD is a PKA target in vivo. Myocytes expressing GFP-PKD were treated with ISO, Forsk or vehicle prior to PKD immunoprecipitation (IP) using GFP antibody and protein A/G magnetic beads. Fig 4B shows a representative IP probed with pRRXS*/T* and GFP antibodies. The GFP panel shows that equivalent amounts of PKD were immunoprecipitated and the control IPs, using beads or control IgG (Fig 4C), show the specificity of the pull-down. ISO and Forsk treatment increased the pRRXS*/T* signal in the myocyte lysate (pre-IP lanes) indicating increased PKA phosphorylation. The pRRXS*/T* signal in immunoprecipitated PKD was likewise increased, indicating PKA phosphorylates PKD.
Figure 4. PKA-dependent phosphorylation of PKD.
(A) In vitro phosphorylation of purified PKD with the catalytic subunit of PKA. Phosphorylation is measured with 2 different PKA consensus motif antibodies (RRXS*/T* top and RXXS*/T* bottom). (B) ISO- and Forsk-induced phosphorylation of immunoprecipitated GFP-PKD1 measured with a PKA consensus motif antibody (RRXS*/T*). Blots are probed with GFP for loading control. (C) Control immunoprecipitations (beads only and control IgG) demonstrate specificity of the PKD pull-down. (D) Potential PKA phosphorylation sites on mouse PKD1 sequence.
Consensus PKA sites were subsequently identified throughout PKD1 with the Scansite program (http://scansite.mit.edu) to further the notion of direct PKA phosphorylation of PKD. PKD contains several potential PKA substrate sequences (Fig 4D) including several known auto- and trans-phosphorylation sites (S916, S255, S748, S203). Among these, the sequence surrounding S426–S427 is of particular interest since either serine residue could serve as phosphoacceptor, the sequence is entirely conserved among vertebrate species. In addition, S427 was recently identified in vitro as a potential PKA target although no link to changes in PKD localization or catalytic activity was reported.40
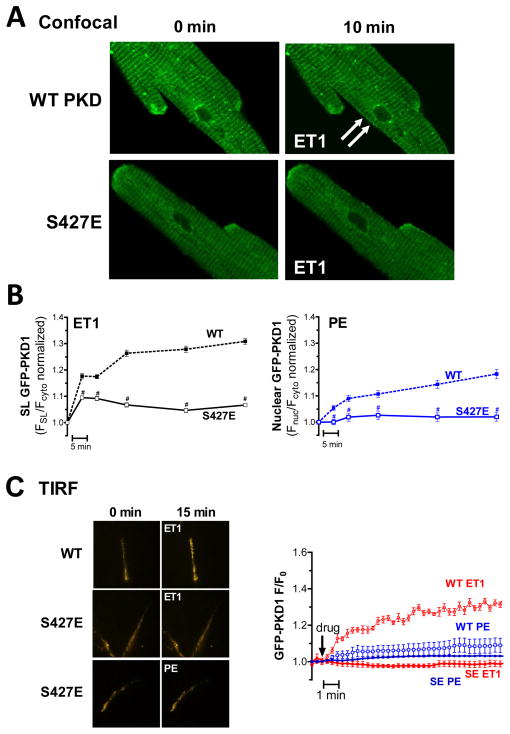
PKD S427E mimics the β-AR inhibition of GqR-induced PKD translocation
We speculated that direct phosphorylation of PKD by PKA at this site, alters PKD translocation. We used confocal microscopy (Fig 5A) to test whether a phosphomimetic substitution at S427 (PKD-S427E) would similarly perturb GqR-evoked PKD translocation. At baseline this mutant had a slightly higher nuclear localization (Fnuc/Fcyto was 0.69±0.02 vs. 0.62±0.02 for WT myocytes) but otherwise targeted like WT (Supplemental Fig V). Our previous findings indicate a predominantly sarcolemmal and nuclear response for ET1 and PE, respectively, so we focused our confocal analysis on the relevant compartment for each agonist. Adult myocytes expressing GFP-tagged PKD-S427E, displayed severely blunted sarcolemmal recruitment in response to ET1 (vs. PKD WT; Fig 5A&B). ET1 application (for 60min) increased FSL/Fcyto by only 7±2% for the S427E mutant vs. 31±4% for PKD-WT. Likewise, PE-triggered nuclear import was drastically reduced in PKD-S427E expressing myocytes vs. WT. Fnuc/Fcyto after 60 min of PE was only 2±2% for PKD-S427E vs. 18±2% for PKD-WT. Parallel TIRF experiments showed even more clearly that S427E substitution resulted in decreased membrane recruitment for both GqR-agonists (Fig 5C). Also of note, following ISO treatment Fnuc/Fcyto was increased (from 0.69±0.02 to 0.77±0.02, similar to WT, Supplemental Fig V), indicating that the β-AR-induced nuclear import of PKD does not depend on S427. However, S427E substitution does recapitulate the PKA-mediated disruption of GqR-induced PKD translocation. Moreover, pS916 measurements show that like ISO pretreatment (Fig 2B), S427E substitution renders PKD resistant to GqR stimuli but not to robust activation with PDBu (Supplemental Fig VI). Together, our data indicate that S427 mediates the PKA-dependent suppression of GqR induced PKD activation and translocation.
Figure 5. S427E substitution mimics β-AR effects on GqR-induced PKD translocation.
(A) Confocal images of GFP-PKD1 WT (top) and S427E (bottom) translocation in response to ET1. (B) Analysis of S427E vs. WT translocation profiles in response to GqR-agonists: ET1-induced sarcolemmal recruitment (left) and PE-induced nuclear import (right) are shown (* P<0.001). (C) TIRF analysis of GFP-PKD S427E vs. WT translocation in response to GqR-agonists (n>6). Representative images are shown on left.
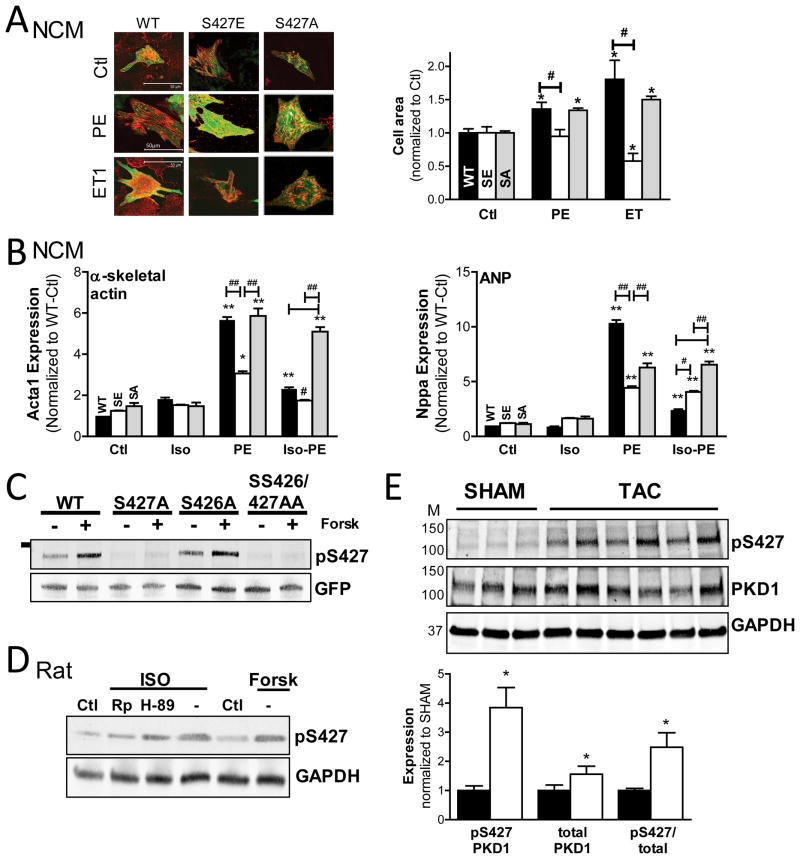
PKD S427 mediates the β-AR effects on downstream GqR-PKD signaling
We speculated that S427E substitution would also affect downstream GqR-induced PKD effects. So in light of the well-characterized role of PKD as an HDAC kinase, we examined the effect of S427E substitution on cardiomyocyte hypertrophy. Neonatal cardiomyocytes (NCMs) were infected with GFP-PKD S427E, S427A or WT. As shown in Fig 6A, expression of PKD-S427E prevented sarcomere assembly and cell enlargement in response to PE or ET1, while S427A responded similar to WT. Cardiac hypertrophy is associated with reactivation of a pathological “fetal” gene program, such as genes encoding for atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and α-skeletal actin. Agonist-dependent elevation of hypertrophy markers can be assessed by RT-PCR. Fig 6B shows that ISO blunts PE-dependent induction of α-skeletal actin expression (left) in NCMs expressing PKD-WT. α-skeletal actin transcript induction by PE was likewise markedly reduced in the presence of PKD-S427E, indicating that S427E substitution recapitulates the β-AR effects on downstream GqR-evoked PKD signaling. Similar results were obtained for ANP, BNP and myosin7 (Fig 6B right and SVII). Additionally, ISO was unable to suppress PE-dependent gene expression for the unphosphorylatable S427A mutant (Fig 6B).
Figure 6. S427E substitution mimics PKA-dependent effects on GqR-PKD signaling.
(A) Confocal images (left) of effect of GFP-PKD1-WT, -S427E and -S427A expression (green) in NCMs on sarcomere assembly (β-actinin:red) and cell enlargement in response to GqR-agonists (quantification right panel). (n=50–100 cells, *P<0.05) (B) RT-PCR analysis of S427E and S427A vs. WT effect on hypertrophic marker induction in NCMs in response to agonist. Results for α-skeletal actin (left) and ANP (right) are shown. (*P<0.05 vs. Ctl, # vs. WT). (C) Test of pS427 antibody using unphosphorylatable GFP-PKD1 variants expressed in HEK293 cells (n=2, GFP=loading control). (D) Measurement of PKD S427 phosphorylation in rat adult cardiomyocytes in response to Forsk or ISO±PKA inhibitors, H89 and Rp-8Br-cAMP (n=2). (E) Western blot analysis of S427 phosphorylation and PKD1 expression in cardiac homogenates from 3 week TAC mice vs. their SHAM-operated controls. Representative blot is shown on the left and quantification of signals on the right.
Altered S427 phosphorylation in cardiac disease
These data indicate that S427 is a key regulatory site on PKD, which can render the kinase resistant to GqR stimuli. Given the role of PKD in cardiac hypertrophy and heart failure development, it is therefore tempting to speculate that the phosphorylation state of this PKD control point would also change during the pathogenesis of heart failure. So we developed a custom antibody to monitor S427 phosphorylation. We verified antibody specificity using unphosphorylatable GFP-PKD1 constructs in HEK cells. Fig 6C shows that immunoreactivity increased following Forsk-treatment for WT and S426A, but not for S427 and SS426/427AA, indicating this novel antibody specifically detects phosphorylation at S427. This antibody signal also increased in lysates from ISO- or Forsk-treated myocytes, but not in the presence of PKA inhibitors H89 or 8-Br-Rp-cAMP (Fig 6D). Subcellular fractionation experiments found that in response to ISO PKD pS427 signals were increased throughout the cell, but particularly in the sarcolemmal compartment (Supplemental Fig VIII). These findings confirm ISO triggers PKA-dependent phosphorylation of S427. We then probed S427 phosphorylation in cardiac homogenates from 3 week TAC mice vs. their SHAM controls. Echocardiography confirmed banded mice had significant alterations in structural and functional cardiac parameters (Supplemental Fig IX). Fig 6E shows that S427 phosphorylation, PKD1 expression and S427-phosphorylated PKD1 were all increased in TAC vs. SHAM. These observations suggest that S427 phosphorylation could be modulated during the development of cardiac hypertrophy and heart failure and deserves further investigation.
DISCUSSION
Here we show for the first time that β-AR/PKA modulation triggers atypical PKD activation by inducing its nuclear activation without preceding sarcolemmal translocation. We also demonstrate that important crosstalk exists between GqR and β-AR signaling and uncover the effects of β-AR signaling on spatiotemporal regulation of PKD. Furthermore, we identify S427 as a novel regulatory phosphorylation site on PKD with a key role in tuning the spatiotemporal dynamics of PKD activity and downstream functional effects. Our study highlights the importance of local activation and regulation for a multifunctional kinase, such as PKD, to achieve its compartmentalized roles and substrate specificity.
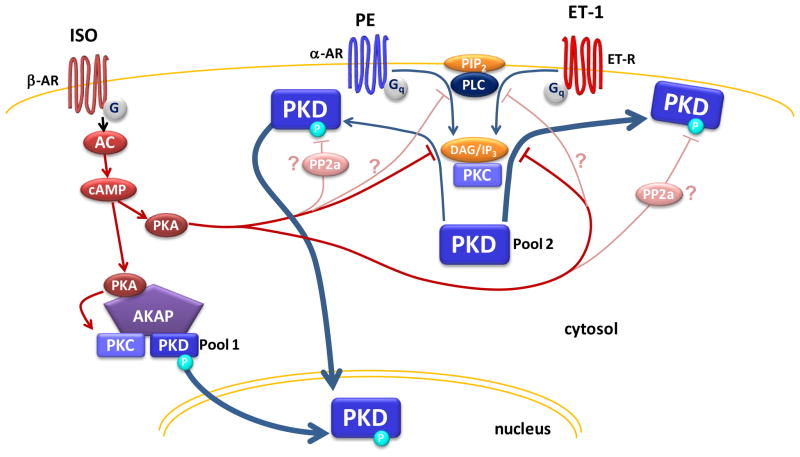
Our data reconcile previous opposing findings of PKA-dependent facilitation29, 30 and inhibition of PKD signaling.32–34 We find here that β-AR/PKA activation of PKD selectively drives nuclear PKD recruitment and activation without initial translocation to the sarcolemma (Fig 1). This differs from the stereotypical activation mechanism. This effect was not detectable with in vitro studies examining global PKD activation via phosphorylation alone (pS916 measurements). This is consistent with prior work using immunoblotting that found no effect of β-AR/PKA stimulation alone on PKD activity.7, 32, 33, 41 The critical advantage of our approach is that it permits in situ monitoring of subtle differences in PKD activity within specific subcellular domains such as the nucleus. Our observation of local, nuclear PKD activation in response to β-AR/PKA signaling fits with reports from the Scott lab29, 30 that PKA activation amplifies PKD signaling. They proposed that AKAP-Lbc serves as a platform synchronizing PKA and PKC activities, thereby facilitating the release of activated PKD and downstream transcriptional activation. The increase in nuclear PKD (and its activity) we see here (Fig 7 left) could originate from this AKAP-Lbc-bound pool of PKD or from another perinuclear AKAP pool (e.g. mAKAP42). Increased PKA activity would trigger the release of activated PKD from this scaffold permitting its movement to the nucleus without prior sarcolemmal recruitment (in this scenario PKA-dependent phosphorylation of S427 does not play a role). Therefore, specific stimuli could engage only a subset of PKD signaling pathway components that are spatially restricted to particular subcellular domains allowing for precise fine-tuning of the functional outcome. The type of data we have obtained should lend valuable insight into the complexities of spatial and temporal regulation of PKD activity.
Figure 7. Working hypothesis of β-AR/PKA modulation of PKD signaling.
For a 1st pool of PKD, β-AR/PKA activation triggers nuclear PKD activity by facilitating activation of PKD at an AKAP scaffold (cyan circle indicates activated PKD). For a 2nd pool of PKD, β-AR/PKA activation prevents GqR-dependent PKD activation and translocation primarily by PKA-dependent phosphorylation of PKD at S427E. We do not exclude that additional mechanisms (such as PLC inhibition or PP2A activation) could contribute to this inhibitory effect of PKA.
Aside from inducing local, nuclear PKD signaling, β-AR/PKA stimulation was also found to prevent GqR-agonist-induced translocation and subsequent PKD activation. Our working hypothesis is that these divergent effects are achieved by modulating distinct pools of PKD in the myocyte (Fig 7) highlighting the importance of precise spatial control of PKD activity. In addition we provide strong evidence that PKA-dependent phosphorylation of PKD at S427 is critical to β-AR dependent modulation of GqR-induced PKD activation and function. This is because the phosphomimetic substitution (PKD-S427E) dramatically reduces GqR-induced PKD translocation and cardiac hypertrophy (Fig 5&6). Therefore our data resolve a long standing question of how this striking β-AR inhibition of PKD activation by GqR agonists occurs. Two other hypotheses had been advanced thus far. First, β-AR-dependent modulation of phospholipase C (PLC) could limit GqR-induced PKD activation (but see also Stangherlin et al.).33, 43–45 Second, PKA stimulation of PP2a activity may abrogate PKD signaling (by terminating activating phosphorylations).46 We do not exclude the possibility that these alternate mechanisms could contribute nor that additional PKA phospho-sites exist on PKD. However, the present study demonstrates that PKA-mediated phosphorylation of PKD at S427 is the principal mechanism of β-AR/PKA inhibition of GqR/PKD signaling.
Phosphoproteomics has identified a multitude of phosphorylation sites on PKD, but only a limited few have known functional consequences.22 Interestingly the predicted PKA target sites on PKD (Fig 5) included known phosphosites such as S748 and S916. However phosphorylation at these sites would be expected to activate, rather than inhibit PKD activity and we did not detect PKA-dependent phosphorylation at these sites in myocyte lysates (Fig 2B).33 Sites S205 and S255 were also less likely based on their known functional effects.11, 47, 48 47. The most interesting candidate was the S427 site just adjacent to the PH domain, i.e. in the position to influence PH-mediated protein interactions and autoinhibitory effects. Indeed, a previous study found that PKA could directly phosphorylate S427 in vitro, but thus far no functional consequences (on localization or activity) had been identified.40 Our experiments firmly establish the S427 site as a key regulatory site of PKD signaling, providing a mechanism to integrate inputs from diverse inciting stimuli: the β-AR and GqR signaling pathways. In this scenario slight changes in PKA activity fine-tune the PKD responsiveness to other stimuli (and possibly which downstream targets are affected). Whether S427 phosphorylation is governed by other positive and negative regulators (kinases/phosphatases), remains to be determined but this site offers intriguing possibilities for modulation of PKD function and cellular phenotype during the pathogenesis of cardiac disease.
Considering the impact of β-AR signaling in the fight-or-flight response and its maladaptive changes in heart failure, the PKA-dependent modulation of PKD signaling efficiency and specificity is likely to have a prominent pathophysiological role. Clearly, further experiments are needed detailing the (β-AR-dependent) modulation of the S427 site vs. alterations in spatiotemporal regulation and function of PKD during different stages of heart failure development. β1-AR desensitization and attenuation of its downstream signaling could reverse the negative modulation of PKD, contributing to the enhanced PKD activity observed in heart failure.18 Other factors such as AKAP expression could influence the topography of PKD signaling (in time and space) in heart failure. Topographic analysis of PKD activity will be key to unraveling the subcellular mechanisms that drive PKD dysfunction in cardiac disease.
In conclusion, we used a fluorescence-centered approach to profile spatial and temporal changes in PKD activation in response to β-AR signaling. This allowed us to visualize local nuclear PKD signaling, undetectable in global PKD measurements. This approach also revealed that the hampered GqR-induced PKD activation following β-AR stimulation, is due to profound perturbations in PKD translocation. PKA-dependent phosphorylation of PKD S427 was found to mediate the perturbation of GqR-induced PKD translocation and function. These findings emphasize the importance of stimuli integration and spatial segregation of PKD in achieving signaling specificity for such a multifunctional kinase. Future studies examining how desensitized β-AR signaling in heart failure contributes to PKD dysregulation, will clarify the role of PKD in cardiac pathophysiology and its potential as a therapeutic target.
Supplementary Material
Novelty and Significance.
What Is Known?
Protein kinase D1 (PKD1) functions as a key transducer of stress stimuli in the events leading up to and during cardiac dysfunction.
Hypertrophic, Gq-coupled receptor agonists (GqR) such as endothelin and phenylephrine robustly activate PKD1.
β-adrenergic (β-AR) signaling through the cAMP/PKA cascade leads to both cardiac protection and cardiac dysfunction.
What New Information Does This Article Contribute?
β-AR signaling prevents GqR driven PKD1 translocation and subsequent activation.
PKA phosphorylates PKD1 at S427, which mediates the β-adrenergic suppressive effects on GqR-triggered PKD1 signaling.
β-AR signaling also selectively induces local nuclear PKD signaling independent of PKDS427 phosphorylation.
PKDS427 phosphorylation is altered during TAC-induced hypertrophy.
PKD1 is a stress-responsive kinase with a key role in pathological cardiac remodeling via phosphorylation of class II histone deacetylases and subsequent activation of MEF2 target genes. It is also well-established that β-AR signaling, as the prime modulator of cardiac contractility and heart rate, is critical for normal and diseased heart function and its dysregulation is a hallmark of heart failure. How β-AR signaling affects PKD1 is controversial. Using a unique fluorescence-based approach our study reconciles these conflicting reports by identifying distinct microdomains of PKD1 signaling in response to β-AR. That is, β-AR triggered local nuclear PKD1 signaling (i.e. activation) andsuppressed GqR-induced PKD1 activation and signaling. The latter effects were mediated via PKA-dependent phosphorylation of PKD1 at S427. Our study identifies PKD1 as a key integration point of the GqR and β-AR signaling pathways. It also identifies S427 as an important regulatory site on PKD1, controlling the spatiotemporal dynamics of PKD activity and downstream functional effects on cardiac hypertrophy and gene expression. Our finding that PKA signaling renders PKD resistant to Gq stimuli provides fundamental insight into how β-AR desensitization in heart failure could contribute to PKD dysregulation and further cardiac dysfunction.
Acknowledgments
The authors would like to thank Dr. Donald M. Bers for his generous support, advice and help in editing this manuscript.
SOURCES OF FUNDING
NIH-R01-HL103933 and American Heart Association Scientist Development Grant 035312N (JB), NIH-P01-HL80105 (DMB), NIH-T32-HL86350 (RRR), NIH-F32-HL112497 (CBN), American Heart Association pre-doctoral fellowship 09PRE2260256 (CWC).
Nonstandard Abbreviations and Acronyms
- ISO
isoproterenol
- Forsk
forskolin
- ET1
endothelin-1
- PE
phenylephrine
- GPCR
G protein coupled receptor
- β-AR
β-adrenergic receptor
- GqR
Gq-coupled receptor
- FRET
Fluorescence resonance energy transfer
- DAG
diacylglyceral
- PH
pleckstrin homology
- NCM
neonatal rat cardiomyocytes
- PDBu
Phorbol 12,13-dibutyrate
- ANP
Atrial natriuretic peptide
- BNP
Brain natriuretic peptide
Footnotes
DISCLOSURES
None.
References
- 1.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKinsey TA. Derepression of pathological cardiac genes by members of the cam kinase superfamily. Cardiovascular research. 2007;73:667–677. doi: 10.1016/j.cardiores.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 3.Bardswell SC, Cuello F, Rowland AJ, Sadayappan S, Robbins J, Gautel M, Walker JW, Kentish JC, Avkiran M. Distinct sarcomeric substrates are responsible for protein kinase d-mediated regulation of cardiac myofilament ca2+ sensitivity and cross-bridge cycling. The Journal of biological chemistry. 2010;285:5674–5682. doi: 10.1074/jbc.M109.066456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuello F, Bardswell SC, Haworth RS, Yin X, Lutz S, Wieland T, Mayr M, Kentish JC, Avkiran M. Protein kinase d selectively targets cardiac troponin i and regulates myofilament ca2+ sensitivity in ventricular myocytes. Circulation research. 2007;100:864–873. doi: 10.1161/01.RES.0000260809.15393.fa. [DOI] [PubMed] [Google Scholar]
- 5.Goodall MH, Wardlow RD, 2nd, Goldblum RR, Ziman A, Lederer WJ, Randall W, Rogers TB. Novel function of cardiac protein kinase d1 as a dynamic regulator of ca2+ sensitivity of contraction. The Journal of biological chemistry. 2010;285:41686–41700. doi: 10.1074/jbc.M110.179648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haworth RS, Cuello F, Herron TJ, Franzen G, Kentish JC, Gautel M, Avkiran M. Protein kinase d is a novel mediator of cardiac troponin i phosphorylation and regulates myofilament function. Circulation research. 2004;95:1091–1099. doi: 10.1161/01.RES.0000149299.34793.3c. [DOI] [PubMed] [Google Scholar]
- 7.Harrison BC, Kim MS, van Rooij E, Plato CF, Papst PJ, Vega RB, McAnally JA, Richardson JA, Bassel-Duby R, Olson EN, McKinsey TA. Regulation of cardiac stress signaling by protein kinase d1. Mol Cell Biol. 2006;26:3875–3888. doi: 10.1128/MCB.26.10.3875-3888.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA. Protein kinases c and d mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24:8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozgen N, Obreztchikova M, Guo J, Elouardighi H, Dorn GW, 2nd, Wilson BA, Steinberg SF. Protein kinase d links gq-coupled receptors to camp response element-binding protein (creb)-ser133 phosphorylation in the heart. The Journal of biological chemistry. 2008;283:17009–17019. doi: 10.1074/jbc.M709851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doppler H, Storz P, Li J, Comb MJ, Toker A. A phosphorylation state-specific antibody recognizes hsp27, a novel substrate of protein kinase d. The Journal of biological chemistry. 2005;280:15013–15019. doi: 10.1074/jbc.C400575200. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Zheng S, Storz P, Min W. Protein kinase d specifically mediates apoptosis signal-regulating kinase 1-jnk signaling induced by h2o2 but not tumor necrosis factor. The Journal of biological chemistry. 2005;280:19036–19044. doi: 10.1074/jbc.M414674200. [DOI] [PubMed] [Google Scholar]
- 12.Storz P, Doppler H, Toker A. Protein kinase d mediates mitochondrion-to-nucleus signaling and detoxification from mitochondrial reactive oxygen species. Mol Cell Biol. 2005;25:8520–8530. doi: 10.1128/MCB.25.19.8520-8530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MS, Wang F, Puthanveetil P, Kewalramani G, Innis S, Marzban L, Steinberg SF, Webber TD, Kieffer TJ, Abrahani A, Rodrigues B. Cleavage of protein kinase d after acute hypoinsulinemia prevents excessive lipoprotein lipase-mediated cardiac triglyceride accumulation. Diabetes. 2009;58:2464–2475. doi: 10.2337/db09-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinbusch LK, Dirkx E, Hoebers NT, Roelants V, Foretz M, Viollet B, Diamant M, van Eys G, Ouwens DM, Bertrand L, Glatz JF, Luiken JJ. Overexpression of amp-activated protein kinase or protein kinase d prevents lipid-induced insulin resistance in cardiomyocytes. Journal of molecular and cellular cardiology. 2013;55:165–173. doi: 10.1016/j.yjmcc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Dirkx E, Schwenk RW, Coumans WA, Hoebers N, Angin Y, Viollet B, Bonen A, van Eys GJ, Glatz JF, Luiken JJ. Protein kinase d1 is essential for contraction-induced glucose uptake but is not involved in fatty acid uptake into cardiomyocytes. The Journal of biological chemistry. 2012;287:5871–5881. doi: 10.1074/jbc.M111.281881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fielitz J, Kim MS, Shelton JM, Qi X, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. Requirement of protein kinase d1 for pathological cardiac remodeling. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3059–3063. doi: 10.1073/pnas.0712265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiang SY, Vanhoutte D, Del Re DP, Purcell NH, Ling H, Banerjee I, Bossuyt J, Lang RA, Zheng Y, Matkovich SJ, Miyamoto S, Molkentin JD, Dorn GW, 2nd, Brown JH. Rhoa protects the mouse heart against ischemia/reperfusion injury. The Journal of clinical investigation. 2011;121:3269–3276. doi: 10.1172/JCI44371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bossuyt J, Helmstadter K, Wu X, Clements-Jewery H, Haworth RS, Avkiran M, Martin JL, Pogwizd SM, Bers DM. Ca2+/calmodulin-dependent protein kinase iidelta and protein kinase d overexpression reinforce the histone deacetylase 5 redistribution in heart failure. Circulation research. 2008;102:695–702. doi: 10.1161/CIRCRESAHA.107.169755. [DOI] [PubMed] [Google Scholar]
- 19.Avkiran M, Rowland AJ, Cuello F, Haworth RS. Protein kinase d in the cardiovascular system: Emerging roles in health and disease. Circulation research. 2008;102:157–163. doi: 10.1161/CIRCRESAHA.107.168211. [DOI] [PubMed] [Google Scholar]
- 20.Rozengurt E, Sinnett-Smith J, Van Lint J, Valverde AM. Protein kinase d (pkd): A novel target for diacylglycerol and phorbol esters. Mutation research. 1995;333:153–160. doi: 10.1016/0027-5107(95)00141-7. [DOI] [PubMed] [Google Scholar]
- 21.Rozengurt E. Protein kinase d signaling: Multiple biological functions in health and disease. Physiology. 2011;26:23–33. doi: 10.1152/physiol.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinberg SF. Regulation of protein kinase d1 activity. Molecular pharmacology. 2012;81:284–291. doi: 10.1124/mol.111.075986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haworth RS, Goss MW, Rozengurt E, Avkiran M. Expression and activity of protein kinase d/protein kinase c mu in myocardium: Evidence for alpha1-adrenergic receptor- and protein kinase c-mediated regulation. Journal of molecular and cellular cardiology. 2000;32:1013–1023. doi: 10.1006/jmcc.2000.1143. [DOI] [PubMed] [Google Scholar]
- 24.Waldron RT, Rey O, Iglesias T, Tugal T, Cantrell D, Rozengurt E. Activation loop ser744 and ser748 in protein kinase d are transphosphorylated in vivo. The Journal of biological chemistry. 2001;276:32606–32615. doi: 10.1074/jbc.M101648200. [DOI] [PubMed] [Google Scholar]
- 25.Waldron RT, Innamorati G, Torres-Marquez ME, Sinnett-Smith J, Rozengurt E. Differential pkc-dependent and -independent pkd activation by g protein alpha subunits of the gq family: Selective stimulation of pkd ser(7)(4)(8) autophosphorylation by galphaq. Cellular signalling. 2012;24:914–921. doi: 10.1016/j.cellsig.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo J, Gertsberg Z, Ozgen N, Sabri A, Steinberg SF. Protein kinase d isoforms are activated in an agonist-specific manner in cardiomyocytes. The Journal of biological chemistry. 2011;286:6500–6509. doi: 10.1074/jbc.M110.208058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bossuyt J, Chang CW, Helmstadter K, Kunkel MT, Newton AC, Campbell KS, Martin JL, Bossuyt S, Robia SL, Bers DM. Spatiotemporally distinct protein kinase d activation in adult cardiomyocytes in response to phenylephrine and endothelin. The Journal of biological chemistry. 2011;286:33390–33400. doi: 10.1074/jbc.M111.246447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erickson JR, Patel R, Ferguson A, Bossuyt J, Bers DM. Fluorescence resonance energy transfer-based sensor camui provides new insight into mechanisms of calcium/calmodulin-dependent protein kinase ii activation in intact cardiomyocytes. Circulation research. 2011;109:729–738. doi: 10.1161/CIRCRESAHA.111.247148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carnegie GK, Smith FD, McConnachie G, Langeberg LK, Scott JD. Akap-lbc nucleates a protein kinase d activation scaffold. Mol Cell. 2004;15:889–899. doi: 10.1016/j.molcel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Carnegie GK, Soughayer J, Smith FD, Pedroja BS, Zhang F, Diviani D, Bristow MR, Kunkel MT, Newton AC, Langeberg LK, Scott JD. Akap-lbc mobilizes a cardiac hypertrophy signaling pathway. Mol Cell. 2008;32:169–179. doi: 10.1016/j.molcel.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spindler MJ, Burmeister BT, Huang Y, Hsiao EC, Salomonis N, Scott MJ, Srivastava D, Carnegie GK, Conklin BR. Akap13 rho-gef and pkd-binding domain deficient mice develop normally but have an abnormal response to beta-adrenergic-induced cardiac hypertrophy. PloS one. 2013;8:e62705. doi: 10.1371/journal.pone.0062705. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Haworth RS, Cuello F, Avkiran M. Regulation by phosphodiesterase isoforms of protein kinase a-mediated attenuation of myocardial protein kinase d activation. Basic research in cardiology. 2011;106:51–63. doi: 10.1007/s00395-010-0116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haworth RS, Roberts NA, Cuello F, Avkiran M. Regulation of protein kinase d activity in adult myocardium: Novel counter-regulatory roles for protein kinase cepsilon and protein kinase a. Journal of molecular and cellular cardiology. 2007;43:686–695. doi: 10.1016/j.yjmcc.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Sucharov CC, Dockstader K, Nunley K, McKinsey TA, Bristow M. Beta-adrenergic receptor stimulation and activation of protein kinase a protect against alpha1-adrenergic-mediated phosphorylation of protein kinase d and histone deacetylase 5. Journal of cardiac failure. 2011;17:592–600. doi: 10.1016/j.cardfail.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bassani RA, Bers DM. Na-ca exchange is required for rest-decay but not for rest-potentiation of twitches in rabbit and rat ventricular myocytes. Journal of molecular and cellular cardiology. 1994;26:1335–1347. doi: 10.1006/jmcc.1994.1152. [DOI] [PubMed] [Google Scholar]
- 36.Mishra S, Gray CB, Miyamoto S, Bers DM, Brown JH. Location matters: Clarifying the concept of nuclear and cytosolic camkii subtypes. Circulation research. 2011;109:1354–1362. doi: 10.1161/CIRCRESAHA.111.248401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nichols CB, Rossow CF, Navedo MF, Westenbroek RE, Catterall WA, Santana LF, McKnight GS. Sympathetic stimulation of adult cardiomyocytes requires association of akap5 with a subpopulation of l-type calcium channels. Circulation research. 2010;107:747–756. doi: 10.1161/CIRCRESAHA.109.216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J, Jr, Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kunkel MT, Toker A, Tsien RY, Newton AC. Calcium-dependent regulation of protein kinase d revealed by a genetically encoded kinase activity reporter. The Journal of biological chemistry. 2007;282:6733–6742. doi: 10.1074/jbc.M608086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith FD, Samelson BK, Scott JD. Discovery of cellular substrates for protein kinase a using a peptide array screening protocol. The Biochemical journal. 2011;438:103–110. doi: 10.1042/BJ20110720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sucharov CC, Dockstader K, Nunley K, McKinsey TA, Bristow M. B-adrenergic receptor stimulation and activation of protein kinase a protect against α1-adrenergic–mediated phosphorylation of protein kinase d and histone deacetylase 5. Journal of cardiac failure. 2011;17:592–600. doi: 10.1016/j.cardfail.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Malik S, Pang J, Wang H, Park KM, Yule DI, Blaxall BC, Smrcka AV. Phospholipase cepsilon hydrolyzes perinuclear phosphatidylinositol 4-phosphate to regulate cardiac hypertrophy. Cell. 2013;153:216–227. doi: 10.1016/j.cell.2013.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu M, Simon MI. Regulation by camp-dependent protein kinease of a g-protein-mediated phospholipase c. Nature. 1996;382:83–87. doi: 10.1038/382083a0. [DOI] [PubMed] [Google Scholar]
- 44.Ali H, Fisher I, Haribabu B, Richardson RM, Snyderman R. Role of phospholipase cbeta3 phosphorylation in the desensitization of cellular responses to platelet-activating factor. The Journal of biological chemistry. 1997;272:11706–11709. doi: 10.1074/jbc.272.18.11706. [DOI] [PubMed] [Google Scholar]
- 45.Mahavadi S, Huang J, Sriwai W, Rao KR, Murthy KS. Cross-regulation of vpac2 receptor internalization by m2 receptors via c-src-mediated phosphorylation of grk2. Regulatory peptides. 2007;139:109–114. doi: 10.1016/j.regpep.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahn J-H, McAvoy T, Rakhilin SV, Nishi A, Greengard P, Nairn AC. Protein kinase a activates protein phosphatase 2a by phosphorylation of the b56δ subunit. Proceedings of the National Academy of Sciences. 2007;104:2979–2984. doi: 10.1073/pnas.0611532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vertommen D, Rider M, Ni Y, Waelkens E, Merlevede W, Vandenheede JR, Van Lint J. Regulation of protein kinase d by multisite phosphorylation. Identification of phosphorylation sites by mass spectrometry and characterization by site-directed mutagenesis. The Journal of biological chemistry. 2000;275:19567–19576. doi: 10.1074/jbc.M001357200. [DOI] [PubMed] [Google Scholar]
- 48.Hausser A, Storz P, Link G, Stoll H, Liu YC, Altman A, Pfizenmaier K, Johannes FJ. Protein kinase c mu is negatively regulated by 14–3–3 signal transduction proteins. The Journal of biological chemistry. 1999;274:9258–9264. doi: 10.1074/jbc.274.14.9258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.