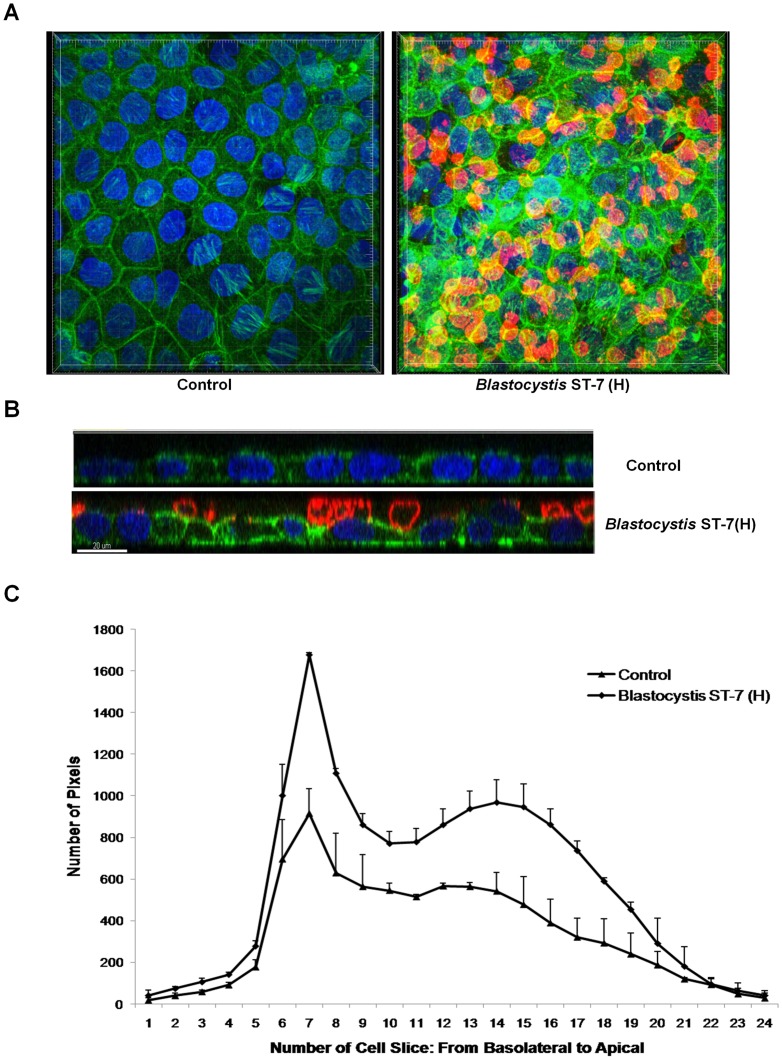
Figure 4. Blastocystis ST-7 isolate H attaches to an intestinal monolayer.
Caco-2 monolayers were grown to confluency on glass coverslips and were then coincubated with Blastocystis ST-7 (H). Normal culture media was used as the negative control. (A) Representative confocal micrographs illustrating attachment of Blastocystis ST-7 (H) to Caco-2 monolayers. Blastocystis was labelled with legumain antibody-mAb1D5and secondary AlexaFluro 594 goat anti-mouse IgM (red). Phalloidin-FITC (green) was used to label F-actin of Caco-2 cells and DAPI (blue) for nuclei. Compared with the negative control, attachment of Blastocystis ST-7 to Caco-2 apical side could be seen clearly. (B) Expanded section views of epithelium of control and ST-7 (H)-treated-monolayers. Parasites could be seen intimately adhering to the epithelium and it could also be noted that the parasites adhered preferentially to the cell-cell junction site (yellow arrows) and induced an increase in actin polymerization. Compared with the negative control which displayed a properly organized epithelium, there was loss of cellular symmetry in the cells treated by ST-7 (H) (black arrowheads). Scale bar = 20 µm. (C) Quantification of F-actin staining in Blastocystis-infected Caco-2 monolayers. Each cell slice (1–25) corresponds to series of images from Z-stack sections taken at 1 µm thickness through the cell monolayer. X-axis illustrates cell layers from basolateral to apical. Y-axis illustrates the number of pixels present over the entire area of image. Monolayers treated with ST-7 (H) resulted in marked increase in actin intensity compared to the negative control (p<0.01).