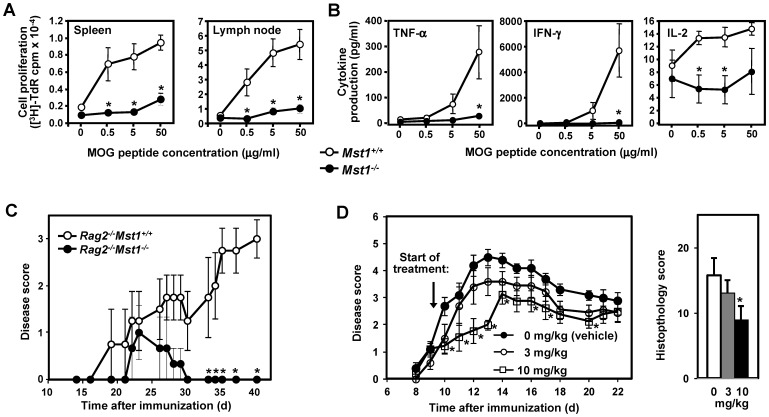
Figure 8. Mst1−/− mice exhibit blunted MOG-specific proliferative and effector cytokine recall responses in vivo.
Splenocytes and lymph node cells isolated from MOGp35–55-immunized Mst1−/− and WT mice (day 11 after immunization; n = 5 per genotype) were incubated with MOGp35–55 at the indicated concentrations. (A) Cell proliferation was assessed by [3H]-thymidine incorporation after 72 hrs of stimulation. (B) Cytokine production was examined 48 hrs after stimulation with MOGp35–55. The results shown are expressed as in Fig. 2 and are representative of two independent experiments. (C) Clinical EAE scores in Rag2−/− mice reconstituted with WT (n = 5) or Mst1−/− (n = 5) CD4+ T cells. Purified WT or Mst1−/− CD4+ T cells were transferred into Rag2−/− mice, which were subsequently immunized with MOG35–55 in CFA. Data are presented as in Figure 7A and are representative of two experiments. (D) Mice treated with an Mst1 inhibitor exhibit decreased severity of EAE. EAE was induced in C57Bl/6-Albino/129SvEv mice (n = 10 per treatment group) by immunization with MOGp35–55 in CFA and treated twice daily with the indicated doses of the Mst1 inhibitor LP-945706 administered by oral gavage in citrate buffed (pH 4.5, 0.1 M) 1% Tween 80 vehicle, starting one day after the onset of disease in 50% of the animals. Mean EAE scores ± SEM (plotted against time after immunization) and aggregate histopathology scores (day 22 after immunization) were analyzed for statistical differences between treatment groups using the Kruskal-Wallis non-parametric analysis of variance (ANOVA) with Dunnett's multiple-comparison post hoc analysis (* p<0.05 compared to vehicle control). Data are representative of two experiments.