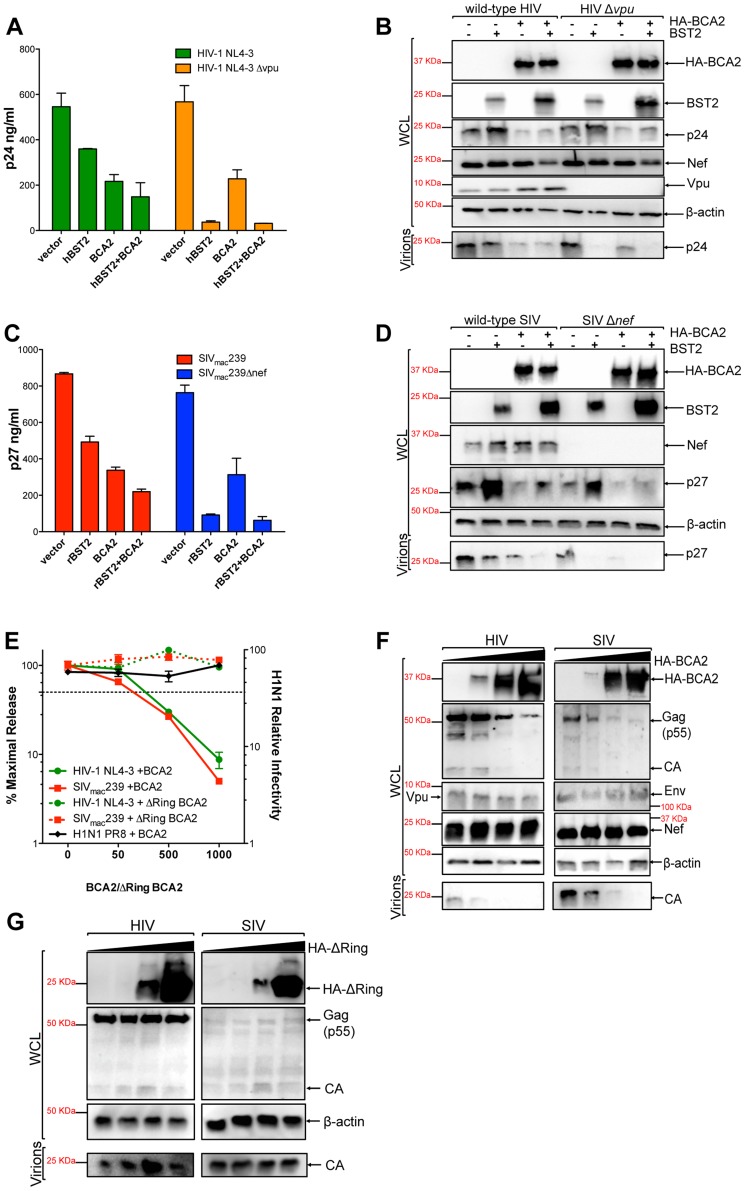
Figure 1. BCA2 has tetherin-independent antiviral activity.
The antiviral activity of BCA2 was tested by co-transfecting 293T cells in duplicate with HIV-1 NL4-3, HIV-1 NL4-3 Δvpu, SIVmac239 or SIVmac239 Δnef proviral DNA in the presence of constructs coding for human or rhesus tetherin (hBST2, rBST2), HA-BCA2 or empty vector. Differences in DNA concentrations were offset by the addition of empty vector, and results were confirmed in three additional independent experiments. (A) Virus release for HIV-1 was measured by p24 antigen-capture ELISA 48 hours post-transfection. (B) The whole cell lysates (WCL) and virions generated from these transfections were analyzed by western blot to assess the expression levels of tetherin, HA-BCA2 and viral proteins (Nef, Vpu, Gag, CA: p24). (C) Virus release for SIV was assessed by p27 antigen-capture ELISA. (D) The expression levels of tetherin, HA-BCA2 and viral proteins were also assessed by western blot (Nef, Gag, CA: p27). (E) 293T cells were co-transfected with equal amounts of HIV-1 or SIV proviral DNA and increasing concentrations of expression vectors coding for HA-BCA2 or ΔRing BCA2. Differences in DNA concentration were offset by adding empty vector. Virus release was determined as described above, and expressed as the percentage of maximal virus release in the absence of HA-BCA2 or the ΔRing BCA2 mutant. To determine the infectivity of influenza H1N1, 293T cells were infected with 1 ml of H1N1 virions produced from HA-BCA2-expressing cells. Infectivity was determined 24 hours post-infection by measuring the amount of influenza hemagglutinin protein (HA) protein present on the cell surface. Similar to panels B and D, whole cell lysates and virions generated from transfections with HA-BCA2 (F) and ΔRing BCA2 (G) were analyzed by western blot. Error bars represent standard deviation of independent experiments.