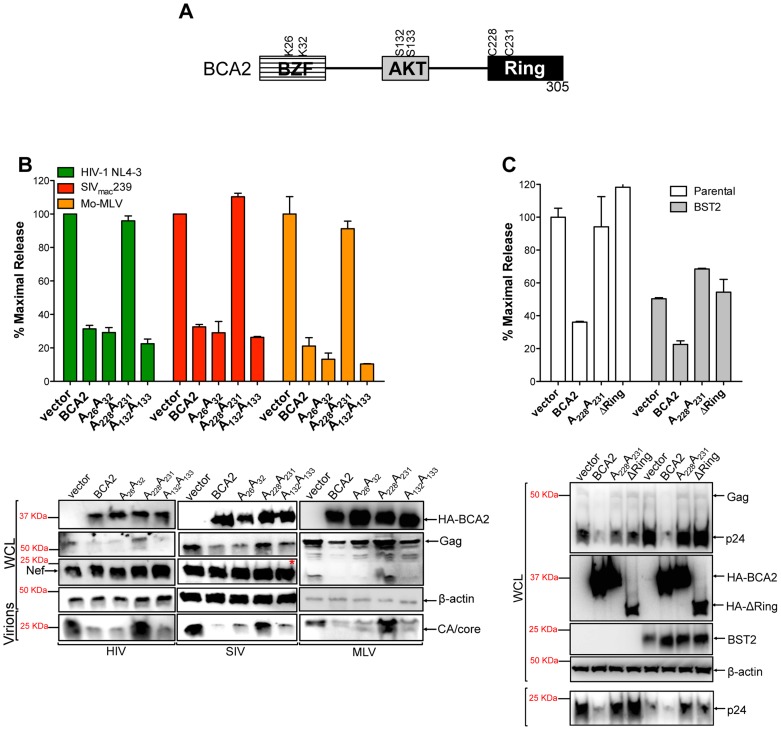
Figure 2. The E3 ligase activity of BCA2 is required for antiviral activity.
(A) Schematic representation of BCA2 with the N-terminal BCA2 Zinc-finger domain (BZF), the AKT-phosphorylation sites, and the C-terminal RING-finger domain. Key residues are indicated. (B) Virus release assays for HIV-1 NL4-3, SIVmac239 and Mo-MLV were performed from cells expressing wild-type HA-BCA2 or the indicated BCA2 mutants, and expressed as the percentage of maximal release, as previously described. The whole cell lysates (WCL) and the virions produced were also analyzed by western blot to assess HA-BCA2, Gag, Nef, CA and β-actin expression. Red asterisk indicates MW of 37 KDa. (C) The tetherin-independent and tetherin-dependent antiviral activity of BCA2 was examined in a virus release assay from parental 293T and 293T cells stably expressing tetherin. Results were also corroborated by western blot of the whole cell lysates (WCL) and pelleted virions. Error bars represent standard deviation of independent experiments.