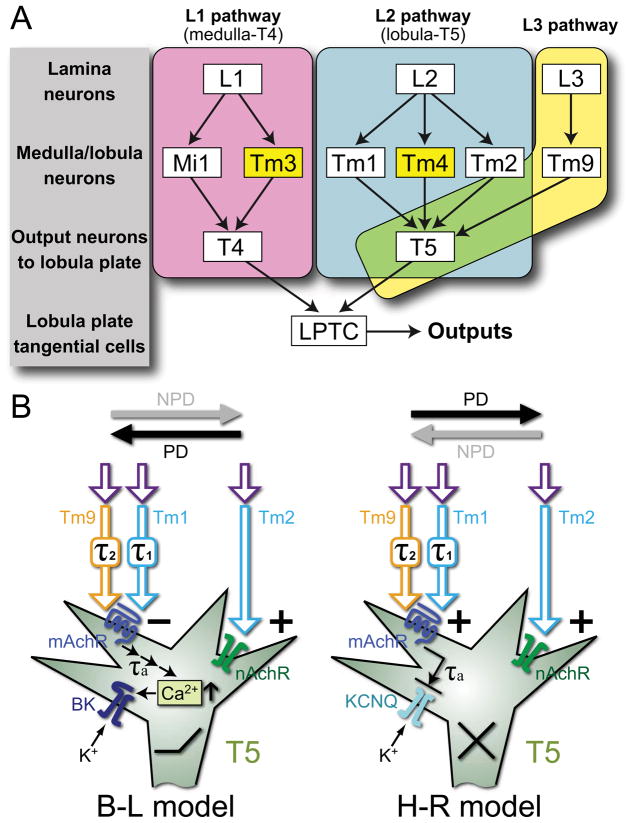
Figure 5.
Models of motion detection pathways and proposed molecular mechanisms. A) Input pathways to T4 and T5 comprise single-column and multi-column (yellow) Tm cells. L1 pathways (magenta) via single-column Mi1 and multi-column Tm3 cells converge upon T4 in the proximal medulla [2]. L2 pathways (cyan) via single-column Tm1 and Tm2 [25] and multi-column Tm4 [2] cells converge upon T5 in the distal lobula, along with Tm9 [2] from the L3 pathway (yellow). T4 and T5 cells in turn provide direction-selective inputs to LPTCs. B) Hypothetical implementation of Barlow-Levick (B–L) and Hassenstein-Reichardt (H–R) models. In both, T5 is proposed to use muscarinic cholinoceptors (mAchR) to receive a delayed signal from Tm1 and Tm9, and nicotinic receptors (nAchR) to receive an instantaneous signal from Tm2. In the B–L model, these two signals converge antagonistically: the activation of nAchR increases sodium conductance and depolarizes the membrane while the activation of mAchR leads to calcium release from internal stores, the activation of a high-conductance calcium-dependent potassium (BK) channel, and eventual membrane hyperpolarization. In the H–R model, the instantaneous and delayed signals interact synergistically: activated mAchR inhibits a Kv type potassium channel, leading to membrane depolarization while activating nAchR depolarizes the membrane. PD: preferred, and NPD: non-preferred directions of motion; τ1, τ2, τa: time delays in Tm1, Tm9, and T5, respectively.