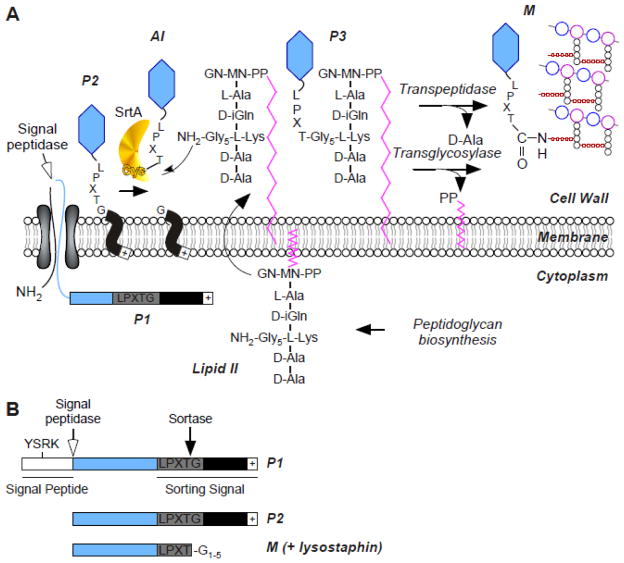
FIG. 1.
Sortase A-dependent surface display of proteins. (A) Model for preprotein secretion and sorting in S. aureus. Surface proteins are first synthesized in the bacterial cytoplasm as full-length precursors (P1) containing an N-terminal signal sequence and a C-terminal sorting signal. The signal sequence directs the cellular export of the polypeptide through the Sec system and, upon translocation, is cleaved by signal peptidase. The product of this reaction, P2 precursor harboring only the C-terminal sorting signal, is retained within the secretory pathway via its C-terminal hydrophobic domain (black box) and positively charged tail (+). Sortase, a membrane anchored transpeptidase with active site cysteine, cleaves the peptide bond between the threonine (T) and the glycine (G) of the LPXTG motif, generating an acyl intermediate (AI). Lipid II, the peptidoglycan biosynthesis precursor, and its pentaglycine crossbridge (Gly5) amino group attacks the acyl intermediate, linking the C-terminal threonine of surface protein to lipid II (P3 precursor) and regenerating the active site of sortase. P3 precursor functions as a substrate for penicillin binding proteins and is incorporated into the cell wall envelope to generate mature anchored surface protein (M) that is also displayed on the bacterial surface. Note that the pentaglycine cell wall crossbridge is not commonly found in other bacteria nonetheless this pathway is conserved in many Gram-positive bacteria where the functional elements of cell wall crossbridges, LPXTG motif, sortase and penicillin binding proteins are conserved (adapted from [188]). (B) Schematic representation of protein precursors identified by using metabolic labeling of staphylococcal cultures with radiolabeled methionine. Immunoprecipitation of labeled polypeptides identifies the precursor P2 and P1. The short-lived AI and P3 intermediates are not identified. Treatment of the staphylococcal peptidoglycan with lysostaphin (glycyl-glycine endopeptidase) releases the final product, i.e. M, the surface protein anchored to the cell wall.