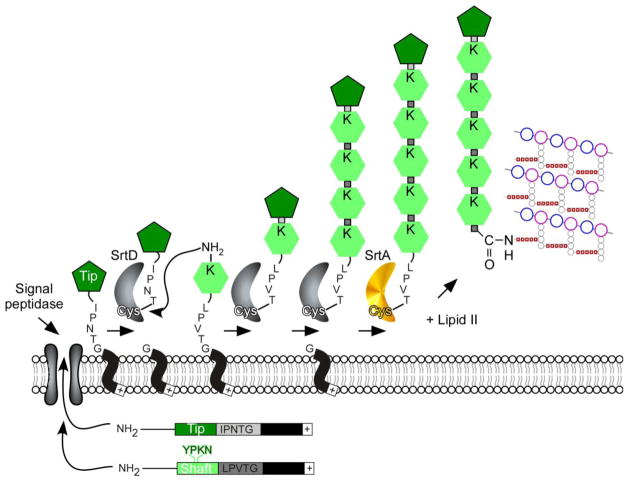
FIG. 3.
Model for sortase-mediated polymerization of heterodimeric pili. Pilin subunits are typical sortase substrates, containing an N-terminal signal peptide that promotes secretion through the Sec system, and a C-terminal cell wall sorting signal. The pilin-specific sortase (SrtC) cleaves the sorting signals of the Tip and Shaft proteins such as the BcpA and BcpB proteins of Bacillus cereus at the Thr of the LPXTG motif, generating an acyl–enzyme intermediate that is resolved by the free amino group of a conserved lysine residue (K) present in the YPKN pilin motif of an incoming pilin subunit. Nucleophilic attack by the side chain of Lys in the YPKN motif results in intermolecular isopeptide bond between tip and shaft. The remainder of the filament assembles by a sequence of similar transpeptidation reactions. Nucleophilic attack of lipid II on the acyl intermediate formed by the housekeeping sortase (SrtA) and the polymerized pilus results in transfer to the cell wall envelope and terminates pilus assembly. Note: a similar mechanism occurs with heterotrimeric pili for example SpaCAB pili of Corynebacterium diphtheriae. SpaC is the pilus tip and SpA, the shaft protein. A third subunit SpaC serves to anchor the polymerized pilus to the cell wall in SrtA-dependent manner (adapted from [169]).