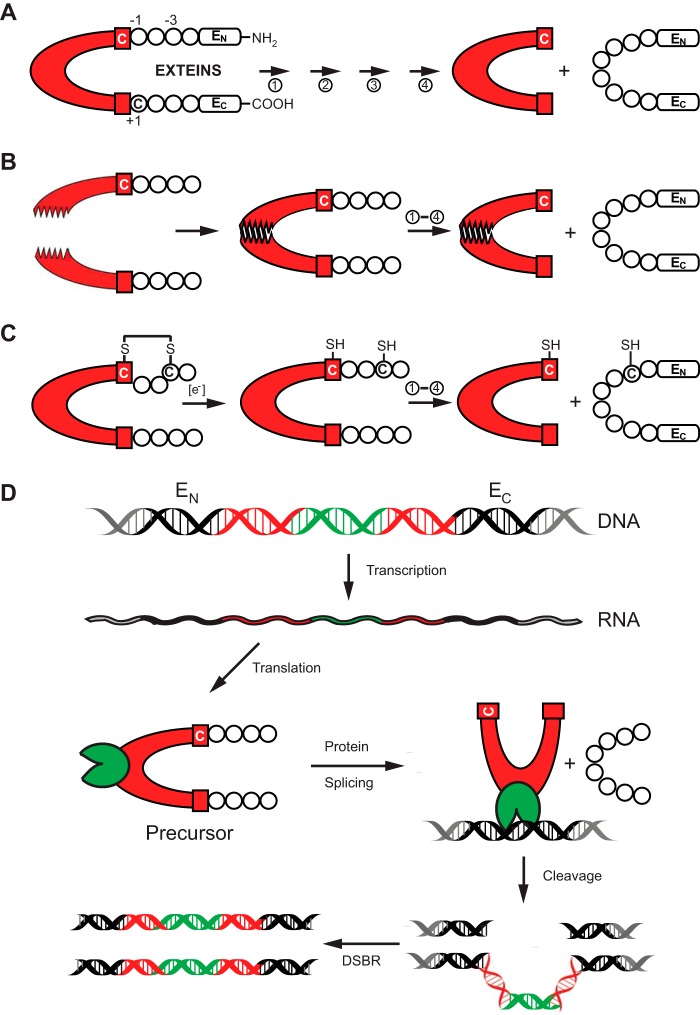
FIGURE 1.
Protein splicing and types of inteins. A, schematic mechanism of protein splicing. The intein is shown in red, flanked by the N-extein (EN) and the C-extein (EC). The four steps of protein splicing are designated by four arrows and are described by Mills et al. (2). The first residue of the intein and that of EC are usually a cysteine, serine, or threonine. They are shown here as Cys1 and Cys+1. B, protein trans-splicing by split inteins. The two halves of the split intein come together via a zipper-like interface. Splicing then proceeds as in A. C, an example of conditional protein splicing. A disulfide bridge, shown between cysteine residues at −3 of EN and at the first residue of the intein (Cys1), prevents protein splicing. In the presence of a reducing reagent, the disulfide bond is broken to release the trapped Cys1 and splicing proceeds. D, HEN-containing inteins are naturally occurring mobile genetic elements. After transcription and translation, precursor undergoes protein splicing with formation of the ligated exteins and intein carrying HEN domain (green). The HEN recognizes and cleaves its cognate intein-less homing site in DNA. The double-strand break is repaired by cellular double-strand break repair (DSBR) machinery using the intein-containing allele as template, resulting in two intron-containing alleles.