FIGURE 2.
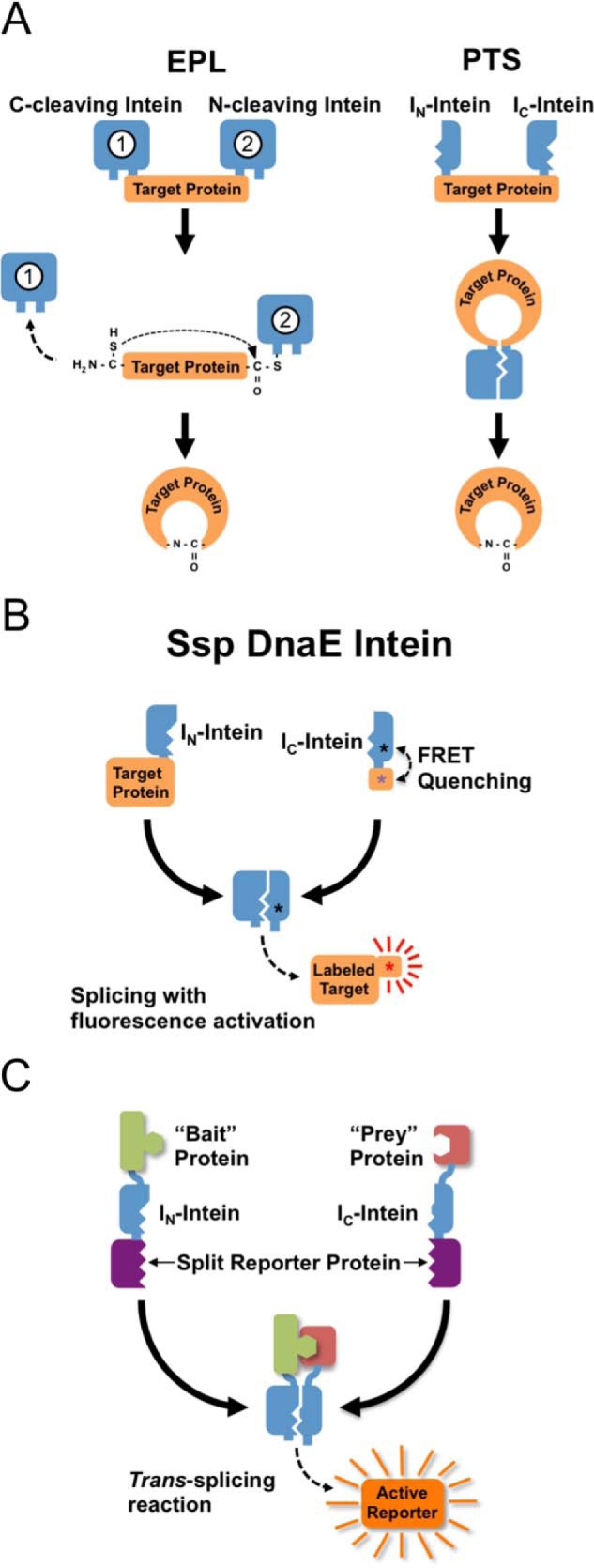
Intein applications involving post-translational modifications of target proteins. A, left panel, EPL methods involve a nucleophilic attack of an N-terminal Cys residue on a thioester formed by a downstream intein. The N-terminal Cys can be generated by a second, upstream intein or by conventional proteolytic cleavage. Right panel, PTS methods produce cyclized proteins through the assembly and splicing of an inverted split intein fused to the N and C terminus of the target protein. B, fluorescent labeling of proteins using a self-quenched intein-based PTS reagent. In this case, PTS simultaneously labels the target protein while releasing the quencher from the dye, thus providing a strong label signal with minimal background from unreacted label. C, protein-protein interactions can be detected by fusing “bait” and “prey” proteins to each half of a weakly interacting trans-splicing intein. Interactions between bait and prey drive assembly and splicing of the intein, resulting in activation of a reporter enzyme.
