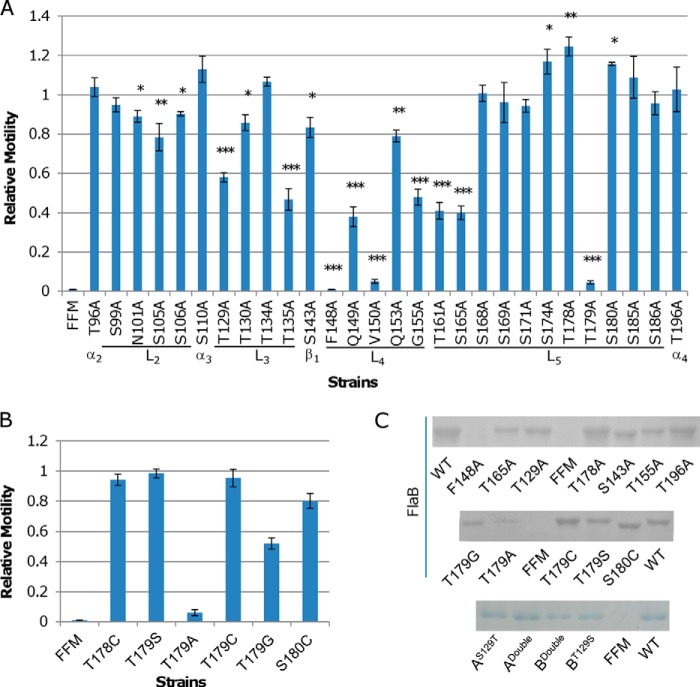
FIGURE 6.
Effect of alanine substitutions in FlaB. A, the importance of 28 sites beyond domains D0 and D1 in motility was evaluated by alanine scanning. The swimming motility of FFM hosting each of the mutant flagellins on 0.25% LB agar plates was assayed. Asterisks, statistically significant difference (*, p < 0.01; **, p < 0.001; ***, p < 0.0001; n ≥ 3). B, to determine whether the hydroxyl group of Thr179 is of significance in motility, the residue was replaced by serine, cysteine, or glycine, and the resulting flagellins were assayed for motility. Residues before and after Thr179 were also examined to reinforce the importance of Thr179. In both A and B, relative motility represents the ratio of mutant FlaB motility to wild type FlaB motility, which is the diameter of the area of motility obtained from the same plate as shown in Fig. 3A, and data are representative of at least three independent experiments with S.D. as the error bar. C, SDS-PAGE analysis of flagellins extracted from the indicated strain. Cultures at the same growing phase (mid-log) were adjusted to the same optical density to ensure that a similar number of cells was used for extraction. FlaBS143A and FlaBS180C mutant proteins migrate faster than others because of the loss of one glycosylation modification. AS129T, ADouble, BDouble, and BT129S refer to Fig. 7A.