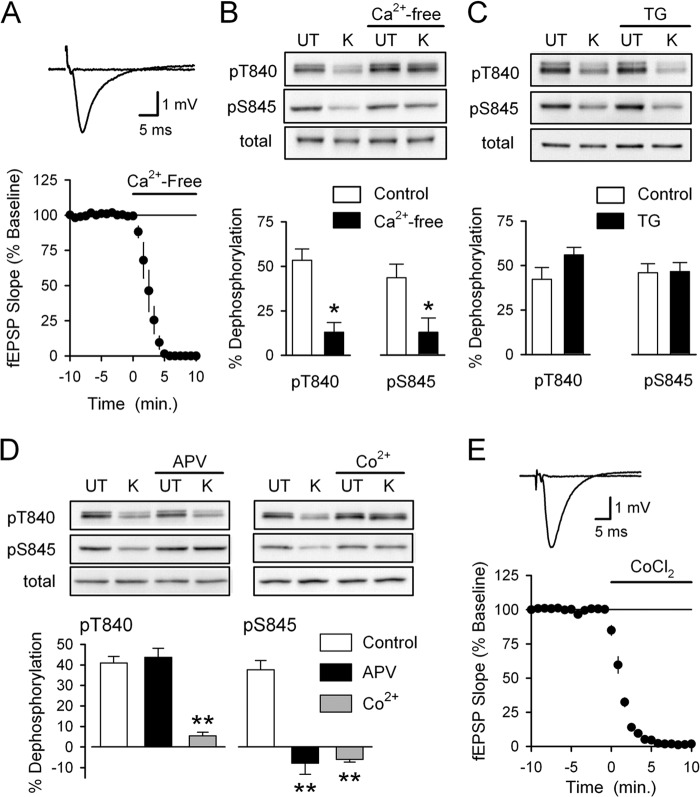
FIGURE 2.
Distinct sources of Ca2+ influx are responsible for depolarization-induced Thr-840 and Ser-845 dephosphorylation. A, a 10-min bath application of Ca2+-free ACSF containing 10 mm EGTA (indicated by the bar) blocks excitatory synaptic transmission (n = 4). Traces show example fEPSPs recorded during base line and after application of Ca2+-free ACSF. B, slices were either untreated (UT) or exposed to high-K+ (K) ACSF in the absence and presence of 10 mm EGTA (Ca2+-free, 10 min pretreatment) (n = 5; *, p < 0.05 compared with % dephosphorylation in absence of EGTA). Ca2+-free ACSF had no effect on basal levels of GluA1 Thr-840 (pT840) and Ser-845 (pS845) phosphorylation or total GluA1 levels. C, inhibiting Ca2+ release from intracellular stores with thapsigargin (TG) had no effect on high-K+ induced GluA1 dephosphorylation at Thr-840 and Ser-845. Slices were pretreated (for 1 h) with ACSF containing either 0.1% DMSO (vehicle control, n = 6) or 2.0–5.0 μm thapsigargin (n = 4 and 2, respectively). Thapsigargin had no effect on total GluA1 levels or basal levels of GluA1 phosphorylation at Thr-840 and Ser-845. D, high-K+ was applied in the absence (control, n = 8) or presence of either 50 μm D-APV (n = 5) or 3 mm CoCl2 (n = 3). Although blocking VGCC with Co2+ inhibited depolarization-induced dephosphorylation at both Thr-840 and Ser-845, blocking NMDARs with APV only prevented dephosphorylation of GluA1 at Ser-845 (**, p < 0.001 compared with % dephosphorylation in controls). APV and CoCl2 had no effect on basal levels of GluA1 phosphorylation at either site, and total GluA1 levels were unchanged in all conditions. E, bath application of ACSF containing 3.0 mm CoCl2 abolishes synaptic transmission (n = 4). Traces show fEPSPs recorded during base line and 10 min after application of ACSF containing CoCl2.