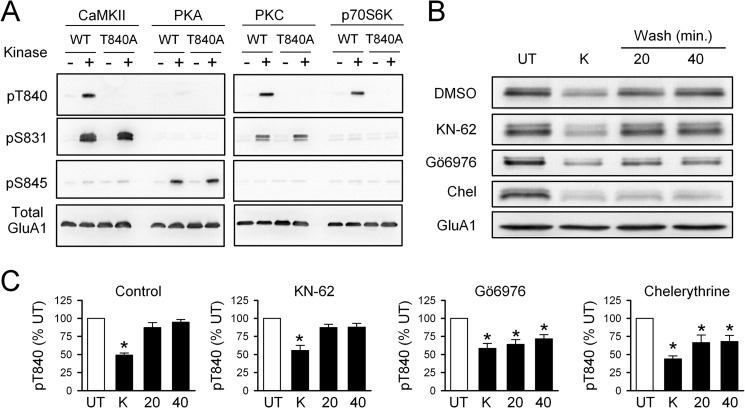
FIGURE 4.
PKC phosphorylates GluA1 at Thr-840 in vitro and in vivo. A, in vitro phosphorylation assay using purified protein kinases and a C-terminal GluA1-GST fusion protein. Thr-840 (pT840) is phosphorylated by CaMKII, PKC, and p70S6 kinase. Phosphorylation by all three protein kinases was disrupted when Thr-840 was mutated to an alanine (T840A). UT, untreated. B, immunoblots show changes in Thr-840 phosphorylation after application of high-K+ (K) ACSF in the continuous presence of different protein kinase inhibitors. Chel, chelerythrine. C, summary of experiments shown in B. Depolarization induced a transient dephosphorylation of AMPAR GluA1 subunits at Thr-840 in vehicle control slices (0.1–0.2% DMSO, n = 7) and in slices exposed to 10 μm KN-62 (n = 4). Thr-840 re-phosphorylation was significantly inhibited in the presence of 10 μm chelerythrine (n = 6) or 2.0 μm Gö6976 (n = 5, *p < 0.05 compared with untreated).