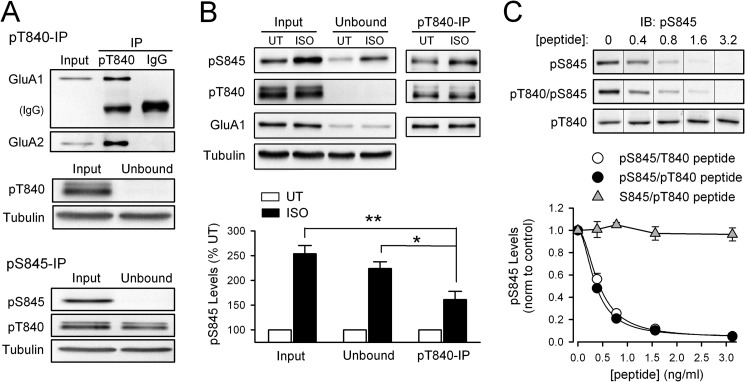
FIGURE 5.
GluA1 Thr-840 phosphorylation inhibits Ser-845 phosphorylation in hippocampal slices. A, homogenates prepared from untreated (NT) hippocampal slices were cleared of phospho-Thr-840 (pT840; top) or phospho-Ser-845 GluA1 (pS845; bottom) by immunoprecipitation (IP) with phospho-specific antibodies (n = 8 for each). IgG, non-immune IgG control immunoprecipitation. Note co-immunoprecipitation of AMPAR GluA2 subunits (top) and the small reduction of Thr-840-phosphorylated GluA1 in the unbound fraction after immunoprecipitation of Ser-845-phosphorylated GluA1 (bottom). UT, untreated. B, PKA activation downstream of β-adrenergic receptor activation preferentially phosphorylates non-Thr-840-phosphorylated GluA1 in hippocampal slices. Anti-phospho-Thr-840 antibodies were used to immunoprecipitate Thr-840-phosphorylated GluA1 (pT840) from lysates prepared from untreated (UT) control slices and slices exposed to isoproterenol (ISO; 1 μm, 3 min) (n = 4). The isoproterenol-induced increase in Ser-845 phosphorylation (pS845) in Thr-840-phosphorylated (pT840) GluA1 (immunoprecipitate fraction) is significantly smaller compared with the increase in Ser-845 phosphorylation seen in both the fraction of GluA1 not phosphorylated at Thr-840 (unbound fraction; *, p < 0.05) and total lysate before immunoprecipitation (input, **, p < 0.01). C, peptide competition assay comparing the ability of Ser-845-phosphorylated (open circles; IC50 = 0.43 ± 0.04 ng/ml) and Ser-845/Thr-840-phosphorylated peptides (filled circles; IC50 = 0.36 ± 0.02 ng/ml) to inhibit binding of phospho-Ser-845 antibody to Ser-845-phosphorylated GluA1 (n = 4 for each, Hill coefficients = 1.9 for both peptides). At the peptide concentrations tested a peptide phosphorylated at Thr-840 alone (triangles, n = 3) had no effect. IB, immunoblot.