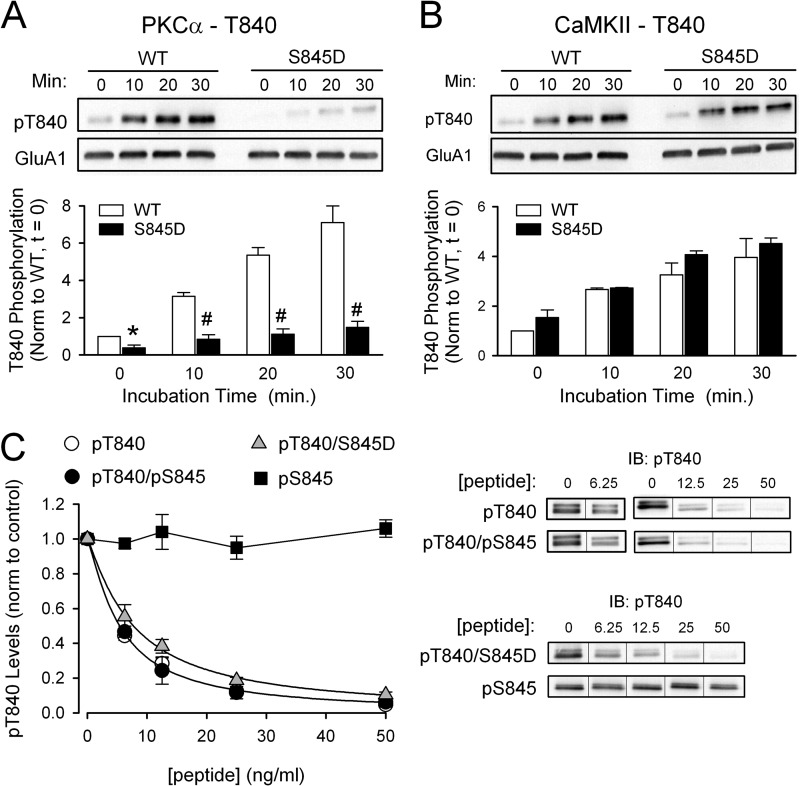
FIGURE 8.
GluA1 Ser-845 phosphorylation inhibits Thr-840 phosphorylation in vitro. A and B, WT and Ser-845 phospho-mimic (S845D) versions of the C-terminal GluA1-GST fusion protein were incubated with purified PKC (A) or CaMKII (B) and immunoblotting with phospho-specific antibodies was used to measure Thr-840 phosphorylation (pT840). Mimicking Ser-845 phosphorylation inhibited Thr-840 phosphorylation by PKC (n = 4; #, p < 0.005 compared with WT GluA1-GST fusion protein) but had no effect on CaMKII phosphorylation of Thr-840 (n = 4). C, phosphopeptide competition assays testing the effects of Ser-845 phosphorylation (filled circles, IC50 = 5.4 ng/ml, n = 4) or substitution of aspartate for Ser-845 (triangles, IC50 = 7.7 ng/ml, n = 4) on the ability of a Thr-840-phosphorylated peptide (open circles, IC50 = 5.4 ng/ml, n = 6) to inhibit phospho-Thr-840 antibody recognition of Thr-840-phosphorylated GluA1. At the concentrations tested a peptide phosphorylated only at Ser-845 (pS845) had no effect (squares, n = 3). IB, immunoblot.