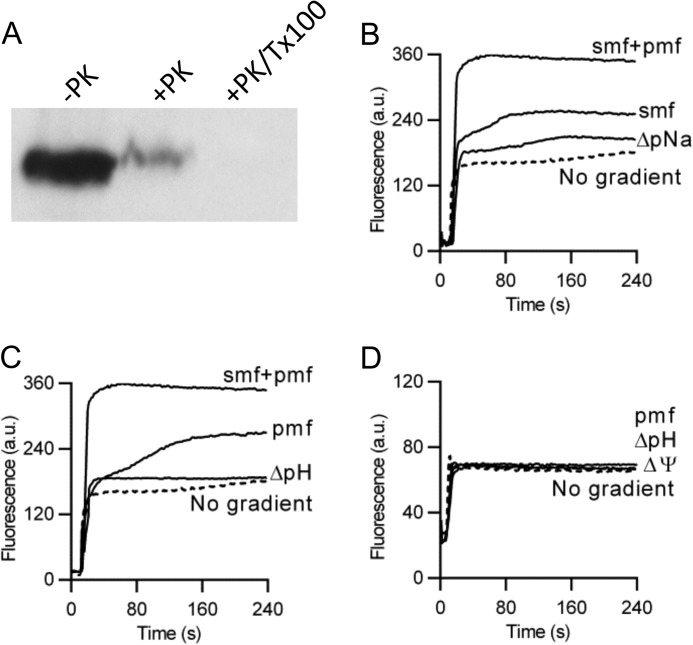
FIGURE 2.
Ethidium transport by NorM-VC in DNA-loaded proteoliposomes. A, availability of the NH2-terminal His10 tag in WT NorM to cleavage by Proteinase K (+PK) at the external side of proteoliposomes compared with control without the protease (−PK). To make the His tag in the liposomal lumen accessible, 1% Triton X-100 was added to the proteoliposomes before proteolysis (+PK/Tx100). Subsequently, the remaining His tag was detected on an immune blot (2.5 μg of protein per lane) probed with anti-His5 antibody. B–D, ethidium transport in proteoliposomes with imposed chemical Na+ gradient (ΔpNa, interior high), sodium-motive force (SMF) (= membrane potential (Δψ)-ZΔpNa, interior positive and high, in which Z ≅ 58 mV at 20 °C), chemical proton gradient (ΔpH, interior acid), proton-motive force (PMF = Δψ-ZΔpH, interior positive and acid), or SMF plus PMF (= Δψ-ZΔpNa-ZΔpH). Experiments with imposed PMF and ΔpH in C were performed in the presence of Na+ ([Na+]in = [Na+]out = 1 mm), whereas those in D were performed in the complete absence of Na+ in plastic containers. The data represent observations in at least three separate experiments using independent batches of purified proteins, cells, and proteoliposomes. Error bars represent the mean ± S.E.