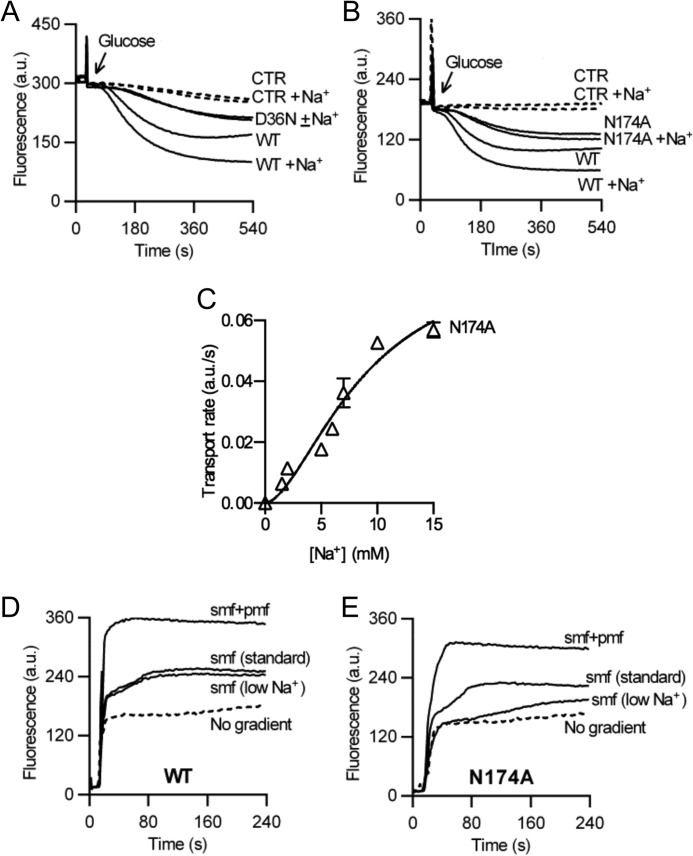
FIGURE 4.
Role of Asp-36 and neighboring residues in Na+ coupling. A and B, in contrast to observations for WT, active ethidium efflux in cells expressing the D36N (A) or N174A (B) mutant was not stimulated by the addition of 1 mm Na+. C, rate of facilitated ethidium efflux as a function of Na+ concentration in ATP-depleted cells containing the N174A mutant demonstrates reduced affinity of the mutant for Na+ compared with WT (compare Fig. 1D). D and E, ethidium transport in DNA-loaded proteoliposomes containing purified WT NorM-VC (D) or N174A protein (E), in which the SMF was imposed artificially. The standard SMF was imposed at [Na+]in/[Na+]out = 100/1 mm, whereas “SMF (low Na+)” was imposed at [Na+]in/[Na+]out = 10/0.1 mm. Traces are based on observations in four separate experiments using independent batches of cells and proteoliposomes. Error bars represent the mean ± S.E.