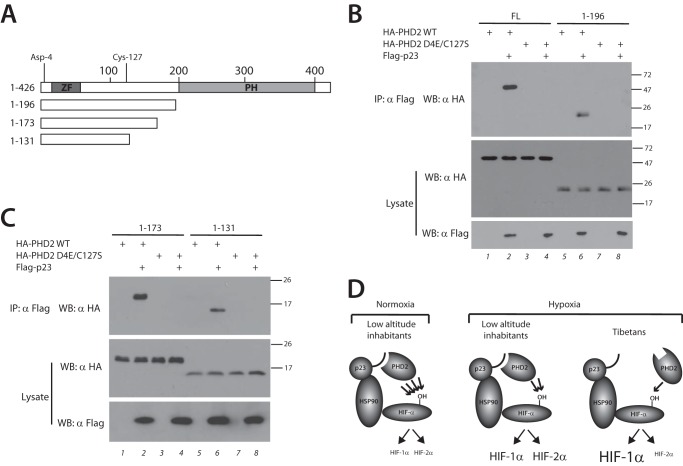
FIGURE 4.
Defective p23 binding localizes to the N-terminal domain of PHD2. A, schematic diagram of PHD2. Zinc finger (ZF) and prolyl hydroxylase domain (PH) are indicated. The numbers at the top indicate residue number, with Asp-4 and Cys-127 as shown. Deletions are as indicated. B and C, HEK293 FT cells were transfected with expression plasmids for the indicated proteins. The cells were lysed and subjected to immunoprecipitation with anti-Flag antibodies, and then the immunoprecipitates and aliquots of lysate were examined by Western blotting as indicated. Positions of molecular mass markers (in kDa) are shown to the right. FL, full length; IP, immunoprecipitation; WB, Western blotting. D, model for Tibetan PHD2 variant. Left panel, under normoxic conditions, WT PHD2 in low altitude inhabitants efficiently prolyl hydroxylates HIF-α (indicated by four arrows), leading to low levels of both HIF-1α and HIF-2α. Middle panel, in response to the chronic hypoxia of high altitude, WT PHD2 less efficiently modifies HIF-α (indicated by two arrows), leading to elevated levels of both HIF-1α and HIF-2α. Right panel, the Tibetan PHD2 is impaired relative to WT PHD2 in its ability to hydroxylate HIF-α (indicated by one arrow), leading to an exaggerated HIF-1α response. However, the Tibetan HIF2A allele likely leads to a blunted HIF-2α response, thereby leading to differential activation of HIF-1α versus HIF-2α.