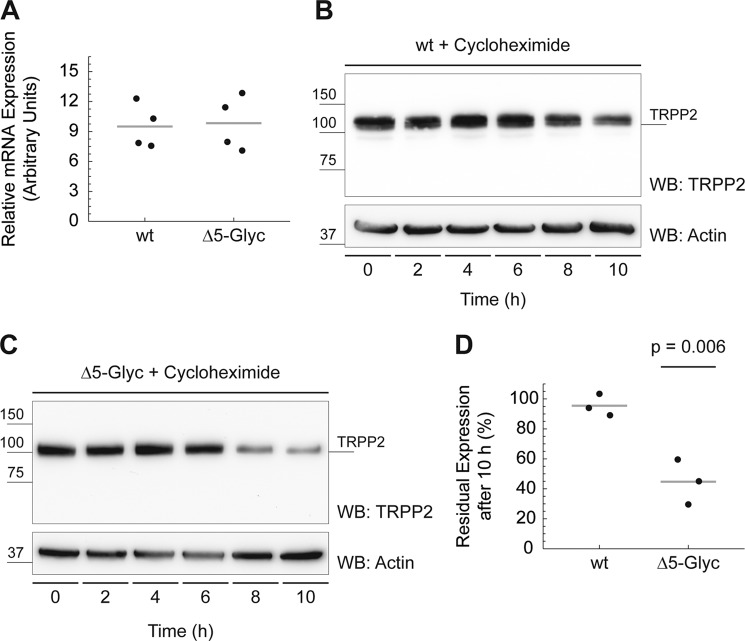
FIGURE 5.
TRPP2Δ5-Glyc protein stability is decreased significantly compared with wild-type TRPP2. A, in transiently transfected HeLa cells, mRNA expression of TRPP2Δ5-Glyc is similar to wild-type TRPP2, as assessed by qPCR. B, wild-type TRPP2 protein stability was probed using cycloheximide, which blocks protein translation. Cells were incubated with 18 μm cycloheximide for 2–10 h, samples were normalized for cell count, and TRPP2 protein persistence was evaluated by Western blot (WB) analysis. β-Actin was used as loading control. C, TRPP2Δ5-Glyc protein stability was probed as described in B. D, group data from B and C. Western blot signal intensities at 0 and 10 h were measured using ImageJ. After 10 h of 18 μm cycloheximide treatment, 95.5% of wild-type TRPP2 was still detected (n = 3). TRPP2Δ5-Glyc, in contrast, shows a significant reduction of protein levels to 44.72% (n = 3, p = 0.006).