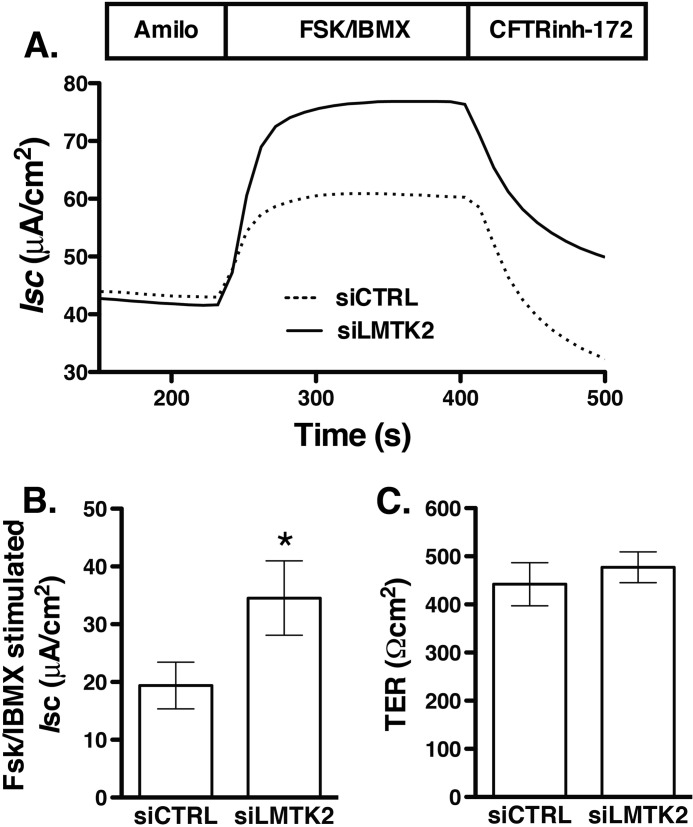
FIGURE 3.
Ussing chamber experiments demonstrating that LMTK2 knockdown increases CFTR mediated Isc across CFBE41o- monolayers. CFBE41o- cells stably expressing WT-CFTR were plated on tissue culture plates and incubated with the optimized transfection mixture containing 50 nm of siRNA specific for LMTK2 (siLMTK2) or the siRNA negative control (siCTRL). After 24 h, cells were trypsinized and plated on collagen-coated Snapwell permeable supports and cultured for an additional 6 days to establish polarized monolayers (total 7 days in culture). CFBE41o- cells were bathed in solutions with apical-to-basolateral Cl− gradient in the presence of amiloride (Amilo, 50 μm) in the apical bath solution to inhibit Na+ absorption through ENaC. Isc was stimulated with forskolin (FSK, 20 μm) and IBMX (50 μm) added to the apical and basolateral bath solution. Thiazolidonone CFTRinh-172 (5 μm) was added to the apical bath solution to inhibit CFTR-mediated Isc. Data are expressed as net stimulated Isc, calculated by subtracting the baseline Isc from the peak stimulated Isc. siCTRL did not affect the forskolin/IBMX-stimulated Isc across CFBE41o-cells compared with the non-transfected cells (data not shown). Representative experiment (A) and summary of data (B) demonstrating that LMTK2 knockdown increased the forskolin/IBMX-stimulated Isc across CFBE41o- cells. siLMTK2 did not change the transepithelial resistance across the monolayers (C). *, p < 0.05 versus siCTRL. 9 monolayers/group from 2 different cultures. Error bars, S.E.