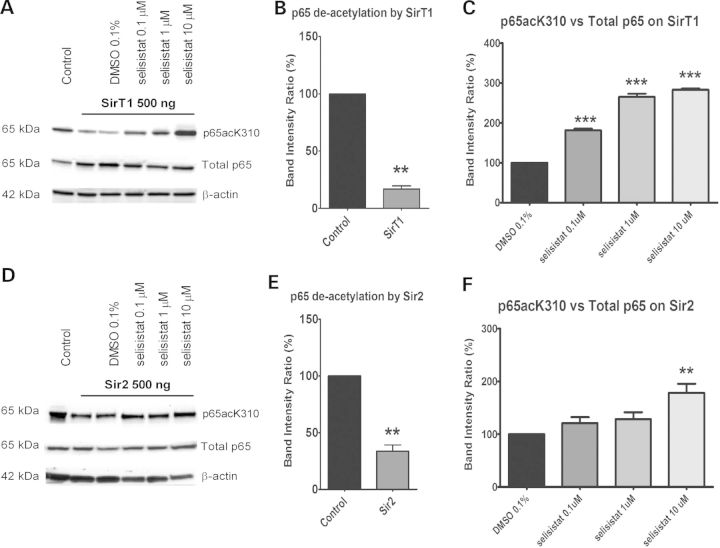
Figure 3.
Selisistat inhibits the deacetylation activity of both human SirT1 and Drosophila Sir2. (A and D) WB analysis of total cell lysates from HEK293 cells transiently transfected with GCN5, p65 subunit and SirT1 (A) or Sir2 (D) (ratio 1: 0.5: 1). p65 acetylation was measured using a specific antibody against acetylated K310 and normalized to the total p65 content. The acetylation level in cells transfected with p65 and GCN5 only served as the control to evaluate the deacetylation activity of SirT1 or Sir2. The acetylation level in cells transfected with p65, GCN5 and SirT1 or Sir2 and treated with DMSO served as the control to evaluate the effect of selisistat treatment. The WBs shown are representative of three independent experiments. (B and E) Band intensity ratios normalized within each independent experiment (n = 3) show the extent of deacetylation by transfected SirT1 (B) or Sir2 (E). p65 deacetylation by SirT1 and Sir2, tested by applying the one-sample t-test versus the theoretical mean of 100, found the samples significantly different from respective controls (**P < 0.01). (C and F) Band intensity ratios normalized within each experiment (n = 3) show the extent to which p65 acetylation is restored by the drug treatment in cells transfected with SirT1 (C) or Sir2 (F). One-way ANOVA analysis followed by the Tukey–Kramer test for multiple comparisons found all concentrations of selisistat tested significantly different from DMSO control in SirT1-transfected cells (***P < 0.005) (C) and selisistat tested at 10 µm was significantly different from DMSO controls in Sir2-transfected cells (**P < 0.01) (F).