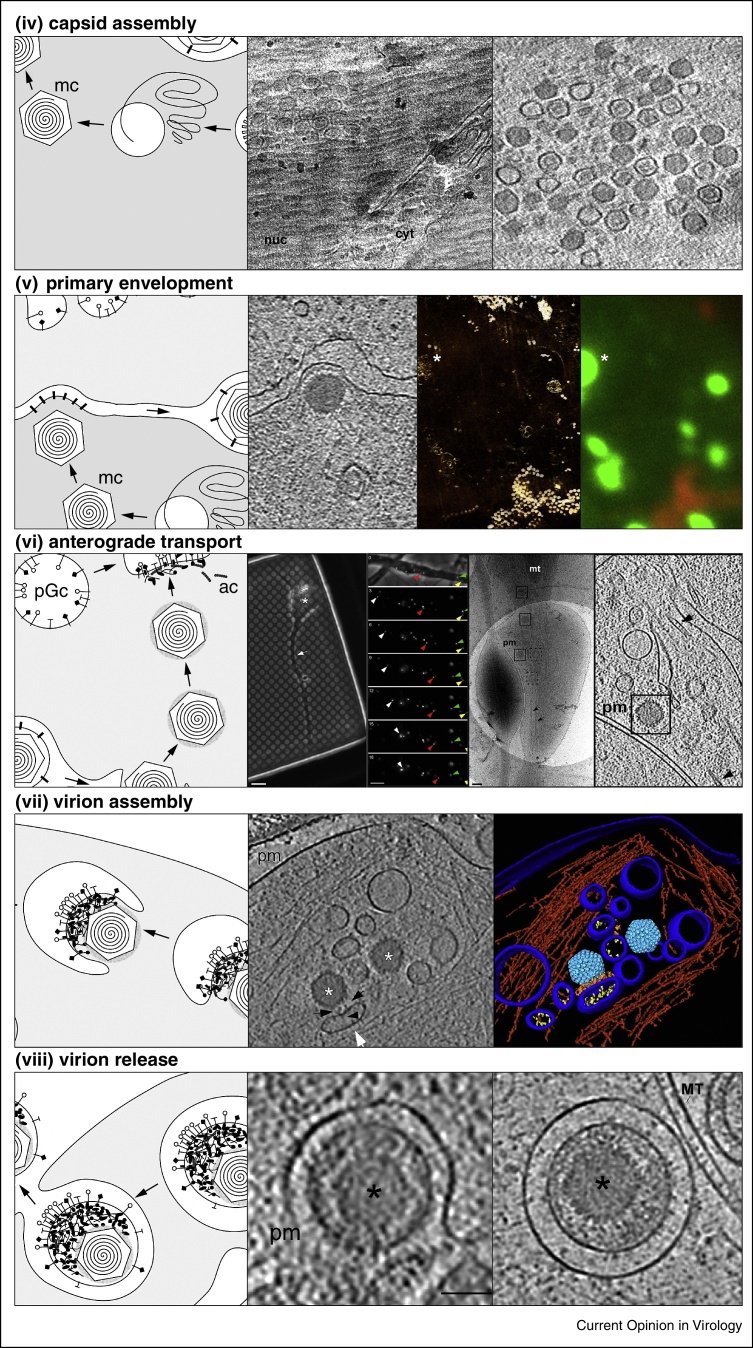
Figure 4.
Dedicated experimental systems used for the study of herpesvirus assembly and transport in vivo. Capsid assembly, electron cryo microscopy of vitreous sections (CEMOVIS) of mammalian cells infected with HSV1 (labelled with GFP) revealed the three distinct types of nucleocapsids close to the inner nuclear membrane (INM) [44]. Shown are a projection image (left) and a slice from a tomogram (right). Primary envelopment, CEMOVIS of mammalian cell infected with murine cytomegalovirus provided snapshots of capsid primary envelopment at the INM (left panel), a layer of density most likely of the nuclear egress complex (NEC) was observed between the capsid and the INM [30]. Correlated imaging with fluorescence and soft X-ray microscopy of cells co-expressing both components of the NEC showed that the NEC is sufficient to drive formation of correctly sized primary envelopes [14]. Anterograde transport, HSV1 infected hippocampal neurons grown directly on holey carbon EM grids were first analysed with live-cell fluorescence imaging that was then correlated with cryoEM and cryoET [48]. Capsids without envelopes were observed undergoing transport along the axon. Virion assembly, secondary envelopment events were observed at axonal terminals [48,49]. Capsids budded into cellular vesicles that clearly were showing viral glycoproteins on their lumenal side and tegument proteins on the membrane side facing the capsid. Shown are a slice from a tomogram (left panel of the cryoEM panels) and 3D rendering of the tomogram (right panel). Virion release, virions transported inside cellular vesicles (right panel) that then fuse at the plasma membrane (left panel) were observed at axon terminals by cryoET [49].