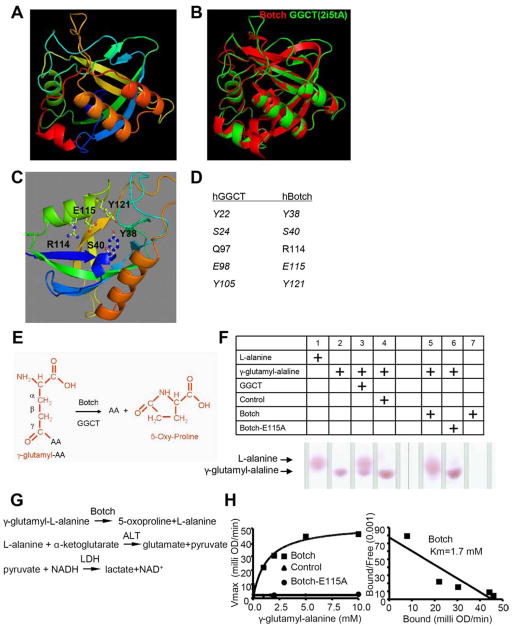
Figure 1. Botch has γ-Glutamyl-Cyclotransferase (GGCT) activity.
(A) Botch structure generated by Swiss-model.
(B) Super-imposition of Botch (Red) structure with human GGCT structure (Green, PDB code 2i5tA) generated by Swiss-model.
(C) Predicted Botch putative active site residues.
(D) Alignment of human Botch putative active site residues with corresponding active site residues of human GGCT.
(E) Schematic representation of GGCT activity. Glutamate that is modified by an amino acid (AA) on the γ carbon is removed by the GGCT activity of Botch liberating and AA and forming 5-oxy-proliine.
(F) Thin layer chromatography of γ-glutamyl-Alanine cleavage by either recombinant GGCT or Botch, but not Botch-E115A.
(G) A schematic diagram of the serial reactions by using oxidation of NADH as the final readout for Botch’s GGCT like activity.
(H) Scatchard plot of quantitative activity of control or Botch or Botch-E115A.
F–H, Experiments were repeated three times with similar results.