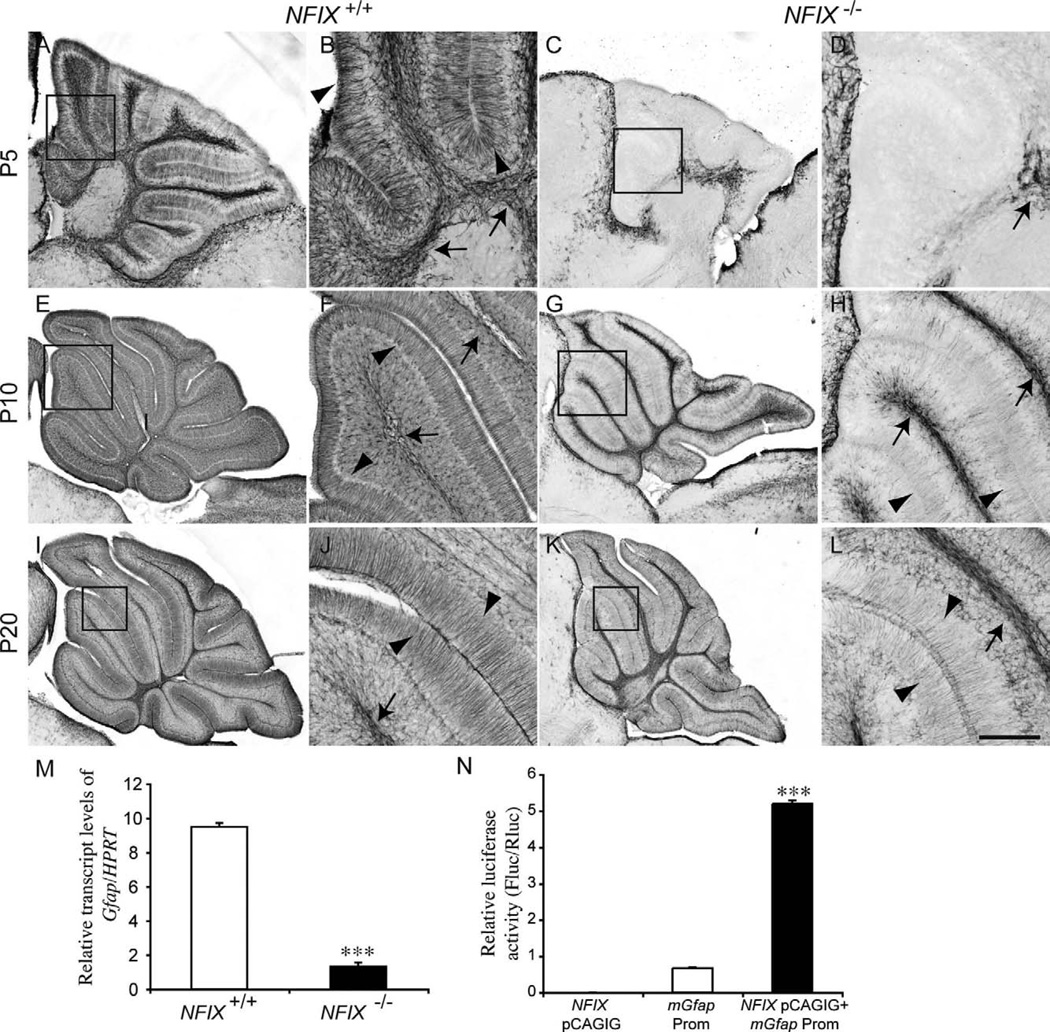
Figure 10.
Delayed glial differentiation in the cerebellum of NFIX−/− mice. Expression of the mature astrocyte marker GFAP at P5 (A–D), P10 (E–H), and P20 (I–L). In the wild type at P5, GFAP expression was evident in both velate protoplasmic astrocytes (arrows in B) and Bergmann glia (arrowheads in B). In the mutant, however, no GFAP expression was detected in the Bergmann glia, and there appeared to be far fewer velate protoplasmic astrocytes in the white matter (arrow in D). At P10 and P20 expression of GFAP within the velate protoplasmic astrocytes (arrows in F,J) and Bergmann glia (arrowheads in F,J) of the wild-type became more pronounced. In the mutant, however, although GFAP expression became more apparent in velate protoplasmic astrocytes (arrows H,L) and Bergmann glia (arrowheads H,L), the level of expression was reduced in comparison with the controls. B,D,F,H,J,L are higher magnification views of the boxed regions (lobules III–V) in A,C,E,G,I,K, respectively. M: qPCR on wild-type and NFIX−/− P5 cerebellar tissue demonstrated significantly reduced levels of Gfap mRNA in the NFIX mutant. N: Transcription reporter assay, demonstrating that expression of NFIX (NFIX pCAGIG) elicits robust transcriptional activation of the reporter gene (firefly luciferase) under the control of the Gfap promoter (Gfap Prom). ***P < 0.001, t-test (M), ANOVA (N). Scale bar = 40 µm in L (applies to J,L); 100 µm for A,C; 25 µm for B,D,F,H; 150 µm for E,G; 200 µm for I,K.