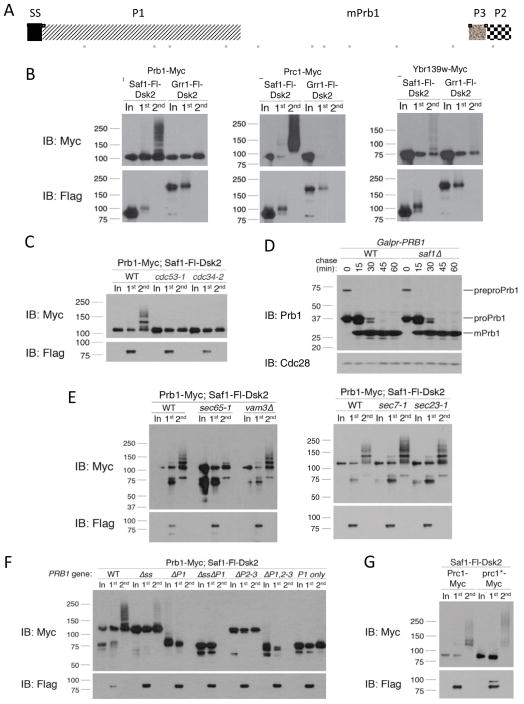
Figure 6. Saf1 targets vacuolar zymogens that fail to properly mature.
(A) Full length Prb1 (preproPrb1) is constituted by 635 amino acids. The N-terminal signal peptide (SP; 20 amino acids) is cleaved during translocation into the ER. In the ER, an intramolecular proteolytic cleavage removes P1 (260 amino acids) to yield proPrb1. In the late Golgi/vacuole, P2 (~30 amino acids) and P3 (~30 amino acids) are cleaved off. Mature Prb1 (mPrb1) is roughly 295 amino acids (~31 kDa). Positions of Prb1 peptides identified by LC-MS/MS analysis of the two-step purification of Saf1-Fl-Dsk2 are represented as solid gray bars below. (B) Two-step purification from extracts expressing Saf1-Fl-Dsk2 and Myc-tagged Prb1, Prc1 or Ykr139w. Grr1-Fl-Dsk2 was used as a negative control for binding specificity. (C) Two-step purification was performed as in (B) in wild type, cdc53-1 or cdc34-2 mutants. Cells were maintained at 23°C then shifted to 38°C for 45 minutes prior to collection. (D) Strains containing PRB1 under the inducible GAL1 promoter were maintained in 2% raffinose and induced with 2% galactose for 15 min, collected and resuspended in 2% glucose. Time points were collected, Western blotted and probed with anti-Prb1 antibody, which recognizes all forms of Prb1. (E) Two-step purification in wild type, sec65-1, vam3Δ, sec7-1 or sec23-1 mutants performed as in (B), except strains were grown/induced at 23°C (permissive temp) and shifted to the 38°C (restrictive temp) for 45 minutes prior to collection. (F) Prb1 constructs, under the control of the TEF1 promoter, were examined by two-step purification as in (B). (G) Two-step purification was performed as in (B) with strains expressing either PRC1 or prc1-G255R, which encodes the Prc1* (aka CPY*) allele.