Abstract
Improvements in chemotherapy and medical support of patients treated with chemotherapy and radiation have led to an ever-increasing number of cancer survivors. Unfortunately, a small fraction of these patients develop secondary hematologic malignancies as a consequence of their exposure to genotoxic anti-cancer regimens. Most of these are myeloid malignancies, therapy-related acute myeloid leukemia (t-AML) or myelodysplasia (t-MDS); however, a small but growing body of literature exists, which describes therapy-related acute lymphoblastic leukemias (t-ALL). Nearly all these cases are reportedly associated with translocations involving chromosome 11q23, the site of the MLL gene. We herein report two cases of ALL occurring after chemotherapy for other malignancies that showed complex karyotypic abnormalities and distinct MLL amplification by fluorescence in situ hybridization analysis. Immunophenotypic analysis showed that both cases expressed a pro-B cell (CD10–) phenotype with aberrant myeloid antigen expression. Although MLL amplification has been reported in therapy-related myeloid disease, to our knowledge this is the first report of MLL amplification occurring in therapy-related B cell ALL.
Keywords: Therapy-related ALL, MLL amplification
Recent advances in cancer treatment have led to an ever-increasing number of cancer survivors. Unfortunately, a small fraction of these patients develop secondary hematologic malignancies as a consequence of exposure to genotoxic anti-cancer regimens (1–4). Most of these are therapy-related myeloid neoplasms, most commonly therapy-related acute myeloid leukemia (t-AML) or myelodysplasia (t-MDS), and less commonly therapy-related myeloproliferative/myelodysplastic neoplasms. Current classification schemes separate therapy-related myeloid disorders into two classes: those related to prior exposure to alkylating agents or radiation and those with prior exposure to DNA topoisomerase II inhibitors (3). The alkylating/radio-therapy group typically have a latency period from time of exposure of 5–7 years and often contain chromosomal abnormalities involving chromosomes 5 and 7. In contrast, those with a history of DNA topoisomerase II inhibitor exposure have a shorter average latency period (1–5 years) and frequently possess chromosomal translocations involving chromosome 11q23, where the MLL gene locus resides. MLL, which stands for “mixed lineage leukemia,” is also frequently rearranged in lymphoblastic leukemias and mixed phenotype acute leukemias (5). Interestingly, MLL also can be altered in myeloid disease through partial tandem duplication or amplification of the genomic region encompassing MLL (amp(MLL)) (6,7). Amp(MLL) of an unrearranged MLL locus has also been described in therapy-related myeloid disease (1,8). Amp(MLL) in therapy-related disease has been associated with complex karyotypic abnormalities, deletion of chromosome arm 5q, TP53 mutations, and prior therapy with alkylating agents (8).
Recently, there has been a growing appreciation that therapy-related leukemias are not limited to the myeloid lineage and that the therapy-related acute lymphoblastic leukemias (t-ALL) make up a small but significant percentage of therapy-related acute leukemias (2,9–15). Translocations involving the MLL locus have been found in a high percentage of t-ALLs. In contrast to t-AML, t-ALLs with 11q23 translocations appear to occur in patients treated with or without DNA topoisomerase II inhibitors (16); however, to our knowledge, amp(MLL) has not been previously reported in t-ALL. We herein report two cases of ALL that showed complex karyotypic abnormalities and distinct MLL amplification by fluorescence in situ hybridization (FISH) analysis occurring after chemotherapy for other malignancies.
Materials and methods
Bone marrow morphology and immunophenotyping
Bone marrow aspirates were prepared and stained using standard Wright-Giemsa methods. Bone marrow samples were also analyzed for immunophenotyping in the Wexner Medical Center at The Ohio State University's Flow Cytometry Laboratory. Samples were analyzed after a lyse/wash procedure according to the manufacturer's specifications (Beckman Coulter, Miami, FL). Mouse anti-human antibodies to human antigens as well as isotype controls were from Beckman Coulter.
Conventional cytogenetic analysis
Cells from bone marrow were cultured in RPMI 1640 media (Fisher Scientific, Houston, TX) supplemented with 2% L-glutamine (Gibco Invitrogen, Carlsbad, CA), 20% fetal bovine serum (Hyclone Laboratories, Logan, TX), and 2% penicillin and streptomycin (Gibco Invitrogen). After culture, cells were harvested, fixed, G-banded using trypsin, and stained with Wright-Giemsa stain. Twenty metaphases were analyzed for each specimen. Karyotypes were described using ISCN 2009 standard nomenclature (17).
Metaphase FISH
Abnormal metaphases were located on previously G-banded slides, and FISH analysis was performed using the Vysis LSI MLL Dual Color Break Apart Rearrangement Probe (Abbott Molecular, Des Plaines, IL) according to the manufacturer's directions.
Results
Patient 1 was an 80-year-old male with a past medical history significant for a stage IV diffuse large B cell lymphoma two years prior. The patient received eight cycles of R-CHOP (rituxan, cyclophosphamide, hydroxydanorubicin, oncovin, prednisone) chemotherapy and achieved a complete remission. One year later, he had a recurrence of his lymphoma in the left humerus and retroperitoneum. He was treated with seven cycles of RICE (rituxan, ifosfamide, carboplatin, etoposide) chemotherapy, after which he received radiation to the involved fields. Eleven months after this treatment, he presented with leukopenia. A bone marrow examination revealed a hypocellular marrow with a marked increase in blasts. Flow cytometry showed that the blasts expressed CD19, CD13, HLA-DR, CD34, TdT, and cytoplasmic CD79a. They lacked expression of CD20, CD10, CD33, CD64, CD65, CD15, myeloperoxidase, and cytoplasmic CD3. Cytogenetic analysis showed a hypodiploid complex karyotype that included a homogeneously staining region (hsr) at 11q23 (Table 1). Metaphase FISH analysis, using an MLL dual-color, break-apart probe (Abbott Molecular), demonstrated amp(MLL) in the hsr at 11q23. Molecular studies at the time of diagnosis were negative for BCR-ABL1 fusions as well as FLT3 mutations.
Table 1.
Karyotypes of Two Patients with amp(MLL)
| Patient 1 |
| 42,XY,−3,hsr(11)(q23),−14,−16,−20[cp6]/43,idem,+mar[cp3]/46,XY[11].ish hsr(11)amp(MLL) |
| Patient 2 |
| 44–45,XY,−5,hsr(11)(q23),−15,+mar1[cp3]/ |
| 48–50,sl,+6,+8,+20,+22[cp2]/ |
| 57−59,sdl1,+1,+del(1)(q12),+2,+7,+10,+hsr(11)(q23)a,+12,+13,+21 ,−mar1 ,+mar 2(cp3)/ |
| 59,sdl2,+X,−1,−del(1)(q12),+del(1)(q25),+5,+10,+11[cp6]/ |
| 73–77,sdl2,+Y,+del(1)(q12),+3,+4,+5,+6,+8,+9,+10,+11,−hsr(11),+12,+13, +14,+15,+16,+17,+17,+18,+18,+19,+22,−mar2,+mar3x2[cp7] .ish hsr(11)amp(MLL),hsr(11)aamp(MLL),mar3 amp(MLL) |
Different from hsr(11 q23) in the stem line, but a normal 11 is still present.
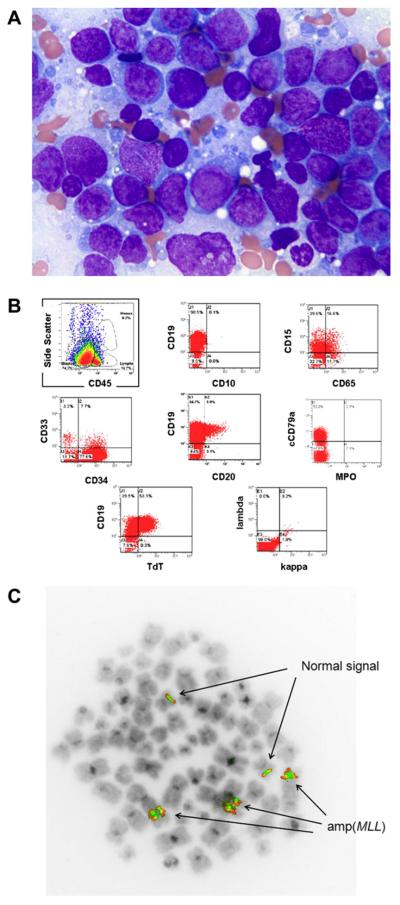
Patient 2 was a 62-year-old male who five years prior had been diagnosed with a pleiomorphic sarcoma of the left hip. The patient received four cycles of doxorubicin and ifosfamide as well as radiation therapy. The patient remained well for five years, but then presented with bleeding gums and a petechial rash on his lower extremities. A complete blood cell count revealed anemia and thrombocytopenia. Bone marrow examination showed a hypercellular bone marrow largely replaced by blasts (Figure 1A). Flow cytometric analysis showed expression of CD19, CD34, CD15, CD65, HLA-DR, cytoplasmic CD79a, and TdT (Figure 1B). The blasts lacked expression of CD10, myeloperoxidase, and cytoplasmic CD3. Cytogenetic analysis showed a very abnormal karyotype with multiple numerical and structural abnormalities (Table 1), including an hsr at 11q23 in the stem line that was duplicated in two side lines, and another different hsr in a marker in one of the side lines. Metaphase FISH analysis showed amp(MLL) on the hsr at 11q23 and on the hsr on the marker chromosomes in one side line (Figure 1C). Molecular studies performed at diagnosis were negative for FLT3 mutations.
Figure 1.
(A) Wright-Giemsa stain of bone marrow from patient 2 showing diffuse sheets of immature blasts. (B) Flow cytometric analysis of bone marrow from patient 2 showing a predominant population of cells with dim CD45 expression and low-light side scatter consistent with blasts expressing a precursor B cell phenotype. (C) Metaphase FISH analysis of patient 2 showing two normal MLL signals as well as three amplified MLL signals.
Discussion
Frequent molecular perturbation makes the MLL locus one of the most common genetic targets in hematologic malignancies, particularly in therapy-related neoplasms. MLL, also known as ALL1 or MLL1, belongs to a highly evolutionarily conserved family of genes known as trithorax genes (5). These are believed to be involved in homeotic specification during development. More recent studies have identified MLL as a member of the SET1 family of histone (H3K4) methyltransferases (18). MLL participates in a macromolecular complex that appears to regulate chromatin modification through methylation, acetylation, and nucleosome remodeling. In general, MLL is believed to act as a co-activator of transcription. One of its important normal functions appears to be involvement in the maintenance of expression of HOX genes, which are critical regulators of hematopoiesis. In hematologic malignancy, the majority of MLL genetic alterations result in translocations involving MLL. These translocations result in fusion proteins with heterologous functions of MLL that result in a block of differentiation (18). Over 100 translocations involve MLL, with at least 70 different partners that have now been described (19). Similarly, amp(MLL), although it does not create an abnormal fusion protein involving MLL, is also considered a “gain of function” mutation that results in increased expression of MLL (20). In contrast to MLL rearrangement, amp(MLL) is a relatively infrequent lesion identified in hematologic cancers (7); however, there seems to be an increased frequency of amp(MLL) in therapy-related myeloid disorders (8). Virtually all amp(MLL) cases reported have been in myeloid disorders. Only a few reports that describe amp(MLL) in precursor lymphoid tumors exist in the literature (21–24). Two of these describe amplification or duplication in B cell lymphoblastic leukemia or lymphoma, and one case involves T cell ALL. No mention is made of prior exposure to chemotherapy for the patients described in these reports. Both of our patients had a hypodiploid stem line, which was also seen in the patient reported by Espinet et al. (21). Interestingly, these authors reported a pro-B cell (CD10–) phenotype in this patient, which was similar to the description of our two patients. This uncommon phenotype is a recurrent feature seen in ALLs with MLL rearrangements (16). The shared phenotype between B cell ALLs with MLL rearrangements and amp(MLL) suggests that both lesions may exert similar influences on the transcriptional programs of these leukemias.
In summary, we report, to our knowledge, the first cases of therapy-related pro-B cell (CD10–) ALL associated with amp(MLL). This observation expands the family of MLL-associated and therapy-related hematologic disorders.
References
- 1.Leone G, Pagano L, Ben-Yehuda D, et al. Therapy-related leukemia and myelodysplasia: susceptibility and incidence. Haematologica. 2007;92:1389–1398. doi: 10.3324/haematol.11034. [DOI] [PubMed] [Google Scholar]
- 2.Bagg A. Therapy-associated lymphoid proliferations. Adv Anat Pathol. 2011;18:199–205. doi: 10.1097/PAP.0b013e31821698ef. [DOI] [PubMed] [Google Scholar]
- 3.Vardiman JW, Arber DA, Brunning R. Acute myeloid leukemia (AML) and related precursor neoplasms. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic Lymphoid Tissues. IARC Press; Lyon, France: 2008. pp. 109–145. [Google Scholar]
- 4.Borowitz MJ, Chan JKC. Precursor lymphoid neoplasms. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic Lymphoid Tissues. IARC Press; Lyon, France: 2008. pp. 171–175. [Google Scholar]
- 5.Hess JL. MLL: a histone methyltransferase disrupted in leukemia. Trends Mol Med. 2004;10:500–507. doi: 10.1016/j.molmed.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Whitman SP, Liu S, Vukosavljevic T, et al. The MLL partial tandem duplication: evidence for recessive gain-of-function in acute myeloid leukemia identifies a novel patient subgroup for molecular-targeted therapy. Blood. 2005;106:345–352. doi: 10.1182/blood-2005-01-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolan M, McGlennen RC, Hirsch B. MLL amplification in myeloid malignancies: clinical, molecular, and cytogenetic findings. Cancer Genet Cytogenet. 2002;134:93–101. doi: 10.1016/s0165-4608(01)00602-1. [DOI] [PubMed] [Google Scholar]
- 8.Andersen MK, Christiansen DH, Kirchhoff M, et al. Duplication or amplification of chromosome band 11q23, including the unrearranged MLL gene, is a recurrent abnormality in therapy-related MDS and AML, and is closely related to mutation of the TP53 gene and to previous therapy with alkylating agents. Genes Chromosomes Cancer. 2001;31:33–41. doi: 10.1002/gcc.1115. [DOI] [PubMed] [Google Scholar]
- 9.Andersen MK, Christiansen DH, Jensen BA, et al. Therapy-related acute lymphoblastic leukaemia with MLL rearrangements following DNA topoisomerase II inhibitors, an increasing problem: report on two new cases and review of the literature since 1992. Br J Haematol. 2001;114:539–543. doi: 10.1046/j.1365-2141.2001.03000.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Wang E, Lu Y, et al. Therapy-related acute lymphoblastic leukemia without 11q23 abnormality: report of six cases and a literature review. Am J Clin Pathol. 2010;133:75–82. doi: 10.1309/AJCPYWC6AQC7BAVJ. [DOI] [PubMed] [Google Scholar]
- 11.Tsuboi K, Komatsu H, Miwa H, et al. T-cell acute lymphoblastic leukemia as a secondary leukemia after a 3-year remission of acute myelocytic leukemia. Int J Hematol. 2003;77:518–521. doi: 10.1007/BF02986622. [DOI] [PubMed] [Google Scholar]
- 12.Bloomfield CD, Archer KJ, Mrozek K, et al. 11q23 balanced chromosome aberrations in treatment-related myelodysplastic syndromes and acute leukemia: report from an international workshop. Genes Chromosomes Cancer. 2002;33:362–378. doi: 10.1002/gcc.10046. [DOI] [PubMed] [Google Scholar]
- 13.Abdulwahab A, Sykes J, Kamel-Reid S, et al. Therapy-related acute lymphoblastic leukemia is more frequent than previously recognized and has a poor prognosis. Cancer. 2012;118:3962–3967. doi: 10.1002/cncr.26735. [DOI] [PubMed] [Google Scholar]
- 14.Pagano L, Pulsoni A, Mele L, et al. Clinical and epidemiological features of acute lymphoblastic leukemia following a previous malignancy. Leuk Lymphoma. 2000;39:465–475. doi: 10.3109/10428190009113377. [DOI] [PubMed] [Google Scholar]
- 15.Tang G, Zuo Z, Thomas DA, et al. Precursor B-acute lymphoblastic leukemia occurring in patients with a history of prior malignancies: is it therapy-related? Haematologica. 2012;97:919–925. doi: 10.3324/haematol.2011.057752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishizawa S, Slovak ML, Popplewell L, et al. High frequency of pro-B acute lymphoblastic leukemia in adults with secondary leukemia with 11q23 abnormalities. Leukemia. 2003;17:1091–1095. doi: 10.1038/sj.leu.2402918. [DOI] [PubMed] [Google Scholar]
- 17.Shaffer LG, Slovak ML, Campbell LJ, editors. ISCN 2009: An International System for Human Cytogenetic Nomenclature. Karger; Basel: 2009. [Google Scholar]
- 18.Slany RK. The molecular biology of mixed lineage leukemia. Haematologica. 2009;94:984–993. doi: 10.3324/haematol.2008.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer C, Kowarz E, Hofmann J, et al. New insights to the MLL recombinome of acute leukemias. Leukemia. 2009;23:1490–1499. doi: 10.1038/leu.2009.33. [DOI] [PubMed] [Google Scholar]
- 20.Hess JL. Mechanisms of transformation by MLL. Crit Rev Eukaryot Gene Expr. 2004;14:235–254. doi: 10.1615/critreveukaryotgeneexpr.v14.i4.10. [DOI] [PubMed] [Google Scholar]
- 21.Espinet B, Florensa L, Salido M, et al. MLL intrachromosomal amplification in a pre-B acute lymphoblastic leukemia. Haematologica. 2003;88:EIM03. [PubMed] [Google Scholar]
- 22.Cuthbert G, Thompson K, McCullough S, et al. MLL amplification in acute leukaemia: a United Kingdom Cancer Cytogenetics Group (UKCCG) study. Leukemia. 2000;14:1885–1891. doi: 10.1038/sj.leu.2401919. [DOI] [PubMed] [Google Scholar]
- 23.Haltrich I, Csoka M, Kovacs G, et al. Six cases of rare gene amplifications and multiple copy of fusion gene in childhood acute lymphoblastic leukemia. Pathol Oncol Res. 2012 doi: 10.1007/s12253-012-9533-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Van Mater D, Goodman BK, Wang E, et al. MLL duplication in a pediatric patient with B-cell lymphoblastic lymphoma. J Pediatr Hematol Oncol. 2012;34:e120–e123. doi: 10.1097/MPH.0b013e3182273b57. [DOI] [PubMed] [Google Scholar]