Abstract
Objective:
To test the effects of lifetime occupational solvent exposure, as measured by dose and timing, on performance on multiple cognitive tests among retired French utility workers.
Methods:
A total of 2,143 retirees in the GAZEL cohort underwent cognitive testing in 2010. Lifetime exposure to chlorinated solvents, petroleum solvents, and benzene was assessed using a job exposure matrix. We modeled effects of lifetime solvent dose, timing of last exposure, and a combination of these metrics on risk for cognitive impairment.
Results:
Thirty-three percent of participants were exposed to chlorinated solvents, 26% to benzene, and 25% to petroleum solvents. High exposure to solvents was significantly associated with poor cognition; for example, those highly exposed to chlorinated solvents were at risk of impairment on the Mini-Mental State Examination (risk ratio 1.18; 95% confidence interval 1.06, 1.31), the Digit Symbol Substitution Test (1.54; 1.31, 1.82), semantic fluency test (1.33; 1.14, 1.55), and the Trail Making Test B (1.49; 1.25, 1.77). Retirees at greatest risk for deficits had both high lifetime exposure to solvents and were last exposed 12 to 30 years before testing. Risk was somewhat elevated among those with high lifetime exposure who were last exposed 31 to 50 years before testing. Those with high, recent exposure exhibited impairment in almost all domains, including those not typically associated with solvent exposure.
Conclusions:
While risk of cognitive impairment among moderately exposed workers may attenuate with time, this may not be fully true for those with higher exposure. This has implications for physicians working with formerly solvent-exposed patients as well as for workplace exposure limit policies.
Occupational exposure to organic solvents—including paints, degreasers, adhesives, and glues—is highly prevalent1 and associated with cognitive impairment.1–4 Solvents affect working memory, attention, and processing speed,5 primarily because of their lipophilic and hydrophilic properties and subsequent ability to be absorbed by fatty tissue and cellular membranes.6
There is a growing body of research about effects of solvent exposure on long-term cognitive function, but few studies with rigorous exposure measures have tested the effects of solvents on multiple domains of cognitive function over a prolonged period. However, some research suggests that effects of high exposure may persist after exposure cessation.4 Many studies use retrospective self-reported exposure assessments with case-control designs,7,8 which may be vulnerable to healthy worker survivor effects or differential reporting of exposures regarding cognitive status.3,5,9 Studies with strong exposure assessment methods are often conducted on previously exposed but nonretired workers or in small samples. While age-related cognitive decline can indeed begin in midlife,10 the extent to which effects of solvents persist into retirement—when normative cognitive aging may also become apparent but occupational exposure has ended—is unknown.
We tested whether lifetime occupational exposure to solvents is associated with cognitive deficits in retired workers by examining the role of lifetime dose, exposure timing, or a combined dose-timing metric in predicting cognitive deficits. We hypothesized that those with high, recent exposure would be at greatest risk but that the highly exposed would exhibit deficits regardless of exposure timing.
METHODS
Study population.
This study used data from GAZEL, a socioeconomically diverse cohort of 20,625 French civil servants employed at the national utility company, Electricite de France–Gaz de France (EDF-GDF), assembled in 1989. Details on cohort recruitment and data collection are available elsewhere.11 Briefly, volunteers' annual self-report questionnaires are linked with occupational exposure histories and in-person health examinations.
In 2010, GAZEL investigators launched a campaign to conduct physical and cognitive examinations of participants at 17 testing centers throughout France. Of the 7,890 who lived in the catchment area of the testing centers and thus were eligible for this study, 3,828 initially responded but only 2,962 of those older than 55 years (the age cutoff for inclusion) attended testing. We excluded participants missing data on retirement status or retirement date (n = 64); solvent exposure (n = 24); cognitive examination (n = 43); and educational attainment (n = 46), a key covariate. Women (n = 643) were excluded from this analysis because of low solvent exposure prevalence (<0.01%). Thus, analyses were conducted on 2,143 men.
Measures.
Outcome.
Neuropsychologists administered a battery of validated French versions of tests parallel to those used in the CONSTANCES cohort.12 General cognitive function was measured using the Mini-Mental State Examination (MMSE)13 and Digit Symbol Substitution Test (DSST)14,15; the latter reflects natural response slowing with age and is sensitive to dementia. Verbal memory was assessed through Free and Cued Selective Reminding Test (FCSRT) short-term and delayed tests.16 Verbal fluency was assessed as both phonemic and semantic fluency.17 Visual-motor speed and executive function were respectively assessed using Trail Making Tests A and B (TMT-A/TMT-B); the latter captures executive function as it involves more complex tasks requiring concentration.18,19 For each test, individuals scoring at or below the bottom quartile of the sample distribution were classified as “impaired,” following conventions of past GAZEL analyses.3,20
Exposure.
A job exposure matrix (JEM) was used to characterize lifetime inhaled exposure to 4 categories of organic solvents: chlorinated solvents (tetrachloromethane, trichloroethylene, perchloroethylene, dichloromethane, trichloroethane), petroleum solvents (hydrazine, others), benzene, and nonbenzene aromatic solvents (toluene diisocyanate). JEMs are a series of tables with exposures listed on one axis and job titles on the other. Titles are grouped to maximize within-group homogeneity, and different tables represent different time periods. Each cell contains an exposure estimate.21 In GAZEL, lifetime exposure trajectories can be calculated because full job histories are available through company records. The present matrix (MATEX) has increased validity because it was constructed for use in EDF-GDF. Exposure estimates by job title and year were derived from ongoing industrial hygiene monitoring of employee workstations and occupational medicine data and extrapolated to workers in similar positions.22,23 Estimates of annual exposure to solvents were calculated as weighted sums of exposure (time-weighted average dose multiplied by exposure probability) for each year between 1960 and 1998.
Individuals were first dichotomized as ever/never exposed to a given solvent type. The exposed were then dichotomized into moderate exposure (total lifetime dose below sample median) and high exposure (lifetime dose at or above median). Exposed individuals were also dichotomized by date of last exposure to a given solvent type: either 1960–1979 (“distal”) or 1980–1998 (“proximal”).
Covariates.
We considered known risk factors for impaired cognitive function potentially associated with solvent exposure: education (measured at GAZEL baseline and dichotomized as high school or below vs more than high school), age in years at testing, occupational grade at midlife (executive, manager, clerk, manual worker; extracted from company records), marital status at year of testing (married/other), weekly units of alcohol consumption (0, 1–14, 15–27, >27), smoking (smoker/nonsmoker), disability (at least one limitation in instrumental activities of daily living),24,25 depressive symptomatology (Center for Epidemiologic Studies–Depression Scale: cutoff = 16),26 body mass index (kg/m2; BMI) (normal weight, overweight, obese), self-reported hypertension (no/yes), and testing center. Marital status and testing center were not retained because of lack of association with outcomes (p > 0.20). Instrumental activities of daily living impairment was excluded because of low prevalence (n = 21). There was close correlation of occupational grade with education, so the former was excluded from final models. The remaining covariates were retained.
Statistical analyses.
We used log-binomial regression to model risk ratios (RR) for impaired cognitive function. Using “unexposed” as the reference, we tested associations between moderate and high exposure to each solvent type and impairment risk for each cognitive score, adjusting sequentially for age and education, and then for the latter plus BMI, hypertension, smoking, alcohol consumption, and depressive symptomatology. We repeated the preceding analyses using time since last exposure (unexposed, distal, proximal) as the exposure metric. We tested for multiplicative interaction between exposure dose and timing by adding an interaction term into adjusted models. We then categorized exposure to each solvent into a 5-level variable to test combined effects of dose and timing: unexposed, moderate-distal exposure, moderate-proximal exposure, high-distal exposure, and high-proximal exposure. We tested associations between these exposure metrics and all cognitive outcomes, using the unexposed as reference. Because aromatic solvent exposure was very rare (<3%), results are not presented and are available on request. We repeated the preceding tests using continuous rather than dichotomous outcomes (available on request). Analyses were conducted using SAS versions 9.2 and 9.3 (SAS Institute, Cary, NC).
Standard protocol approvals, registrations, and patient consents.
Approval for GAZEL was obtained by the French National Committee for Data Privacy (Commission Nationale Informatique et Libertés); our study was conducted with the approval of the human subjects committee at INSERM. Written informed consent was provided by all GAZEL participants at cohort inception (1989) and cognitive testing (2010).
RESULTS
At least one cognitive test was completed by 2,143 men, with average age of 66 years (SD 2.8) and 10 years since retirement at testing. Approximately 33% were ever exposed to chlorinated solvents, 26% ever exposed to benzene, and 25% ever exposed to petroleum solvents (table 1). Twelve percent were highly exposed to one solvent type and 11% to 2 or 3. Within the sample, 8% were smokers, 14% were heavy drinkers, 14% were obese, 37% had hypertension, and 13% had depressive symptoms (results not shown).
Table 1.
Description of exposure and sociodemographic characteristics of the sample, 2010 (N = 2,143)
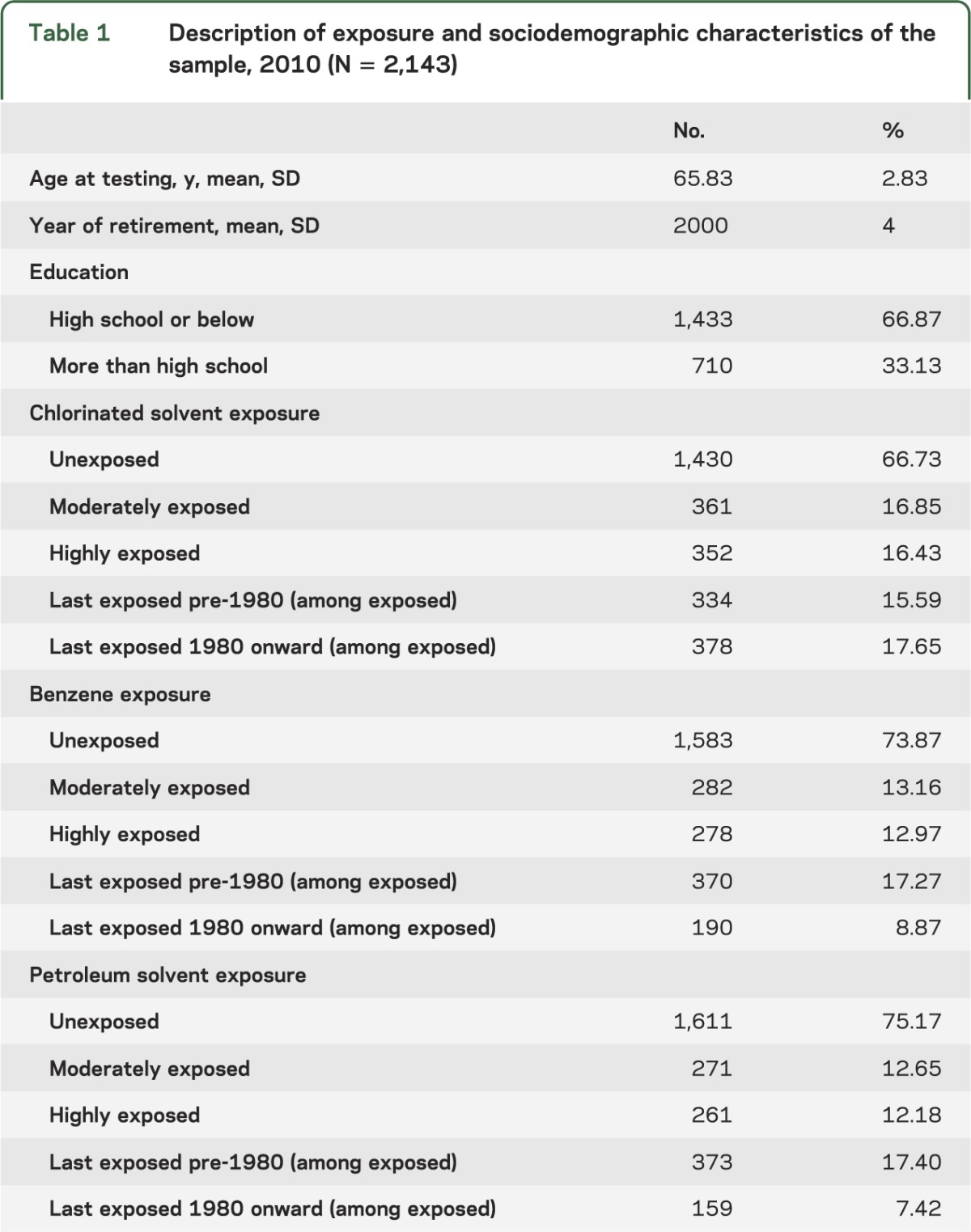
Based on distribution of cognitive scores (table e-1 on the Neurology® Web site at Neurology.org), potential ceiling effects were observed for the MMSE and FCSRT-Immediate and -Delayed; other tests were normally distributed. Eighteen percent had no impaired scores, 59% had 1 to 3 impaired scores, and 22.5% had ≥4 impaired scores.
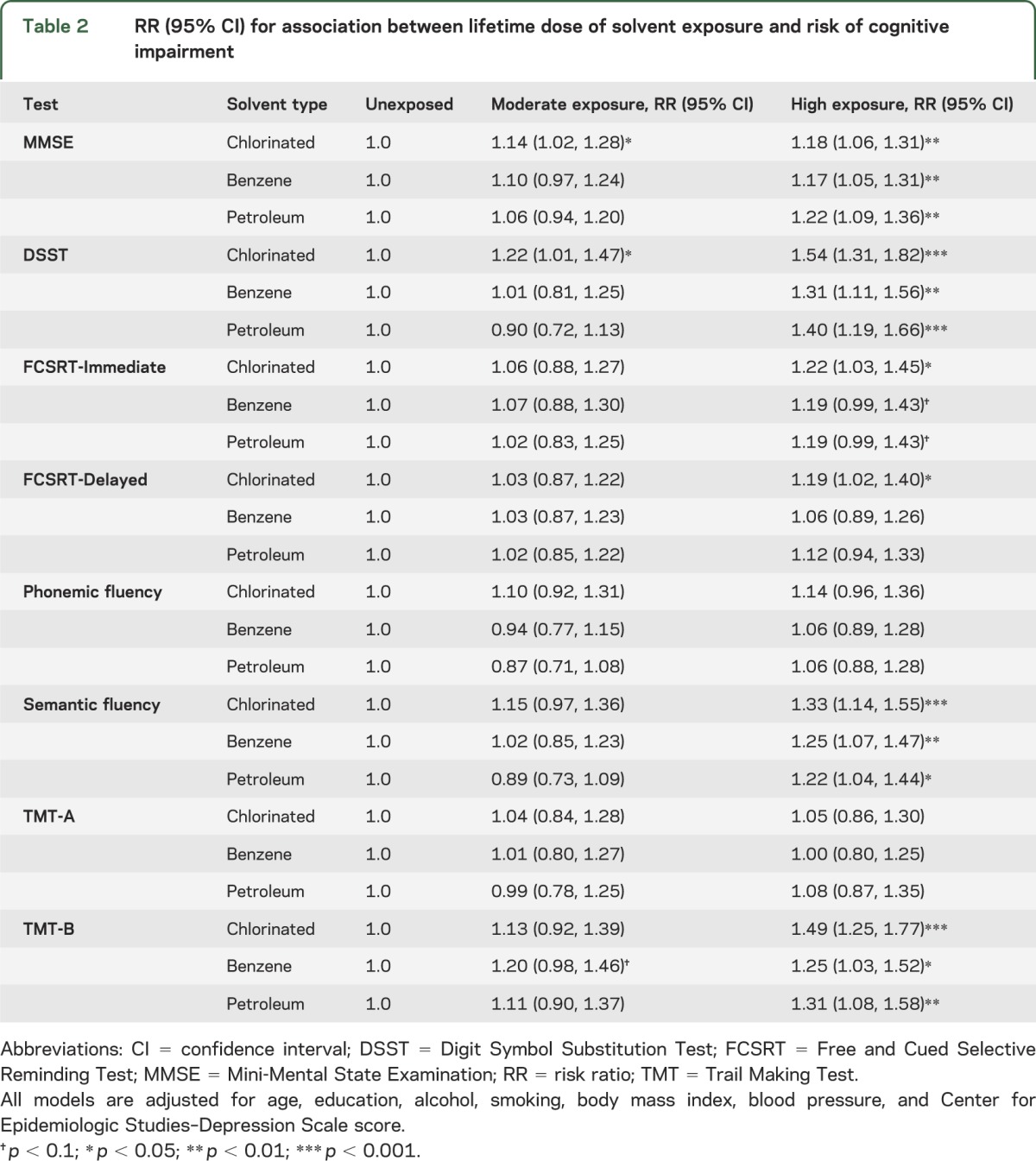
We tested associations between moderate and high exposure to each solvent class and risk of impairment on each test (table 2; reference = unexposed). In adjusted models, impairment on the MMSE was significantly associated with high lifetime exposure to chlorinated solvents (risk ratio 1.18; 95% confidence interval [CI] 1.06, 1.31), benzene (risk ratio 1.17; 95% CI 1.05, 1.31), and petroleum solvents (risk ratio 1.22; 95% CI 1.09, 1.36). Among the moderately exposed, risk was only significantly elevated for chlorinated solvents. We observed similar results in the DSST, although the magnitude of associations was generally higher than for the MMSE. Again, risk of impairment was significantly associated with high exposure to chlorinated solvents (1.54; 1.31, 1.82), benzene (1.31; 1.11, 1.56), and petroleum solvents (1.40; 1.19, 1.66). The other 2 test scores consistently associated with high exposure were semantic fluency and TMT-B. Patterns for these exposures were similar to those for the DSST and MMSE. High exposure was marginally associated with FCSRT-Immediate performance. Solvent exposure was not generally associated with impaired functioning on the FCSRT-Delayed, TMT-A, or phonemic fluency. In sensitivity analyses using continuous rather than dichotomous outcomes, results were similar.
Table 2.
RR (95% CI) for association between lifetime dose of solvent exposure and risk of cognitive impairment
We tested whether exposure timing was associated with poor cognitive functioning; as expected, results showed graded associations between timing and cognitive function in domains similar to those affected by lifetime dose (table e-2). Analysis of combined measures of exposure period and dose (table 3) showed that for MMSE, DSST, semantic fluency, FCSRT-Immediate and -Delayed (except for benzene), and TMT-B, the most hazardous category was high-proximal exposure, which was significantly or marginally associated with increased risk across all solvent types. Some of the latter effects were not evident in separate analyses of dose and timing. High-distal exposure was either significantly or marginally associated with MMSE impairment; this pattern held to a limited extent for associations between chlorinated solvent exposure and DSST. Overall, results suggest that for some cognitive domains (general cognitive status and psychomotor speed), time may not fully attenuate risk when lifetime solvent exposure is high.
Table 3.
RR (95% CI) for associations between combined exposure dose (moderate vs high) and timing (distal vs proximal) and risk of cognitive impairment
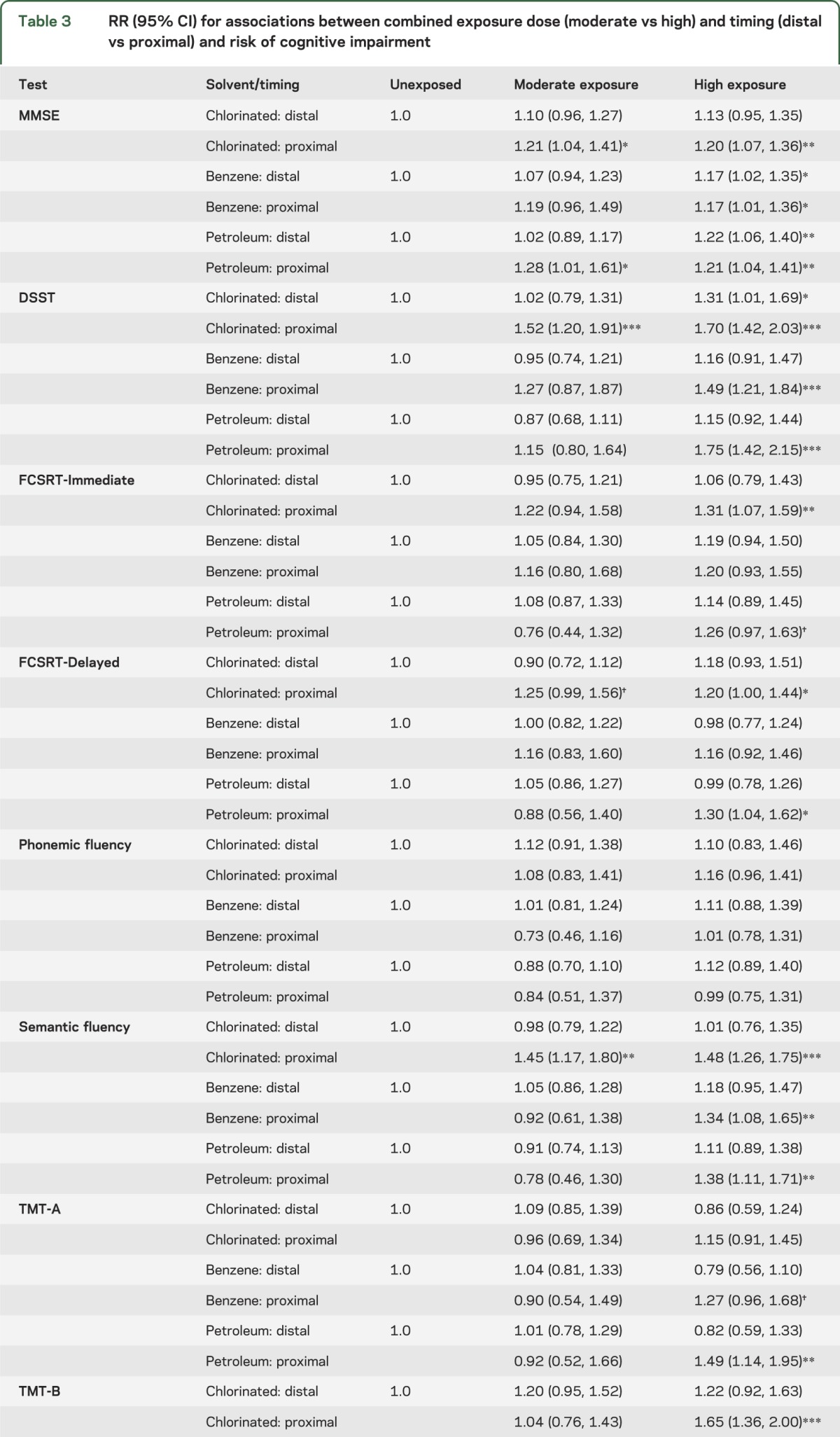
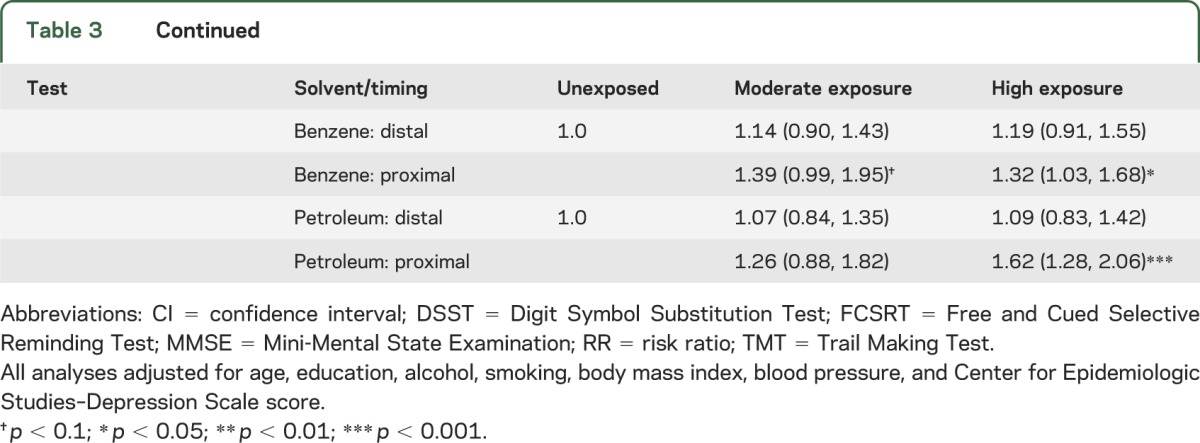
Within dose groups (moderate vs high), those who were proximally exposed to a given solvent almost uniformly had higher risk than the distally exposed, although nearly all CIs overlapped. Within timing groups (distal vs proximal), those who were moderately exposed consistently had lower risk than the highly exposed.
DISCUSSION
We tested associations between lifetime exposure to 4 types of occupational solvents and postretirement risk of poor cognitive function. When quantifying solvent exposure by total lifetime dose, time since most recent exposure, and a combined metric, we found consistent associations between solvent exposure and risk of impaired general cognitive status, psychomotor speed, semantic fluency, immediate recall, and executive function. We did not find consistent associations between solvent exposure and delayed recall, phonemic fluency, or visual-motor speed. Our study advances knowledge of the associations between solvent exposure and cognitive function in several ways: first, by testing effects among workers who had been retired for 10 years on average at the time of testing; second, by expanding the breadth of cognitive tests used and contributing to understanding of domains of long-term function associated with solvent exposure; and third, by elucidating individual and combined roles of solvent timing and dose in shaping risk of poor cognitive function.
We found that associations between multiple types of occupational solvent exposure and certain aspects of cognitive function may exist in retirement, decades after exposure has ceased. A few studies have found effects of high exposure that occurred up to 30 years before testing, although not necessarily in retired populations.9,27,28 Studies using occupational cohorts are prone to healthy worker bias in which exposed workers exit the workforce or retire early, leaving a healthier group remaining.29 Our sample consists of only retired persons, allowing us to show that effects of solvent exposures on cognition may persist many years after labor market exit. In addition, retirement is statutory in EDF-GDF and participants retire at a relatively young age, for 90% between 50 and 60; thus, it is less likely that exposure precipitated retirement.
We expand on prior findings3,20 by explicitly comparing those with more and less recent exposure, allowing simultaneous analysis of dose and recency. We found highest risk of poor cognitive function in those with high, proximal exposure, with a trend toward increased risk among those who were highly but distally exposed. In addition, those in the highest risk category (high-proximal exposure) exhibited cognitive deficits in nearly all tested domains, rather than only domains most frequently associated with solvent exposure. We find that for low-level exposures, effects may indeed attenuate with time, perhaps explaining mixed results in prior studies.5,30
We found consistent associations between lifetime solvent exposure and measures of general cognitive status, psychomotor speed, semantic fluency, and executive function, and marginal associations with immediate recall. While semantic fluency was found to be significantly predicted by solvent exposure, no association was found with phonemic fluency. These 2 fluency tasks involve different cognitive processes, with semantic fluency relying on memory processes and thus temporal lobe structures and phonemic fluency being more sensitive to subcortical lesions such as those seen in vascular dementia or Parkinson disease.31 This pattern of results was also reported in relation to lead exposure.32 A 2008 systematic review of 46 cross-sectional studies of solvent exposure and cognitive function found the strongest decrements in the areas of processing speed and attention, but little effect on verbal memory.4
Our findings are supported by a recent fMRI study that found that occupational solvent exposure was associated with reduced activation in the anterior cingulate cortex, dorsolateral prefrontal cortex, and prefrontal cortex during tests of attention and working memory.33 Because this is the last human brain region to develop, it may be most vulnerable to insults from chemical or physical agents, which may explain its sensitivity to solvent exposure. Furthermore, because prefrontal cortex atrophy is also associated with aging,34 these findings underscore the importance of testing older individuals and retirees to understand whether deterioration happens differentially for those with and without solvent exposures.
Our study has limitations. We had a one-time battery of cognitive tests, administered after the exposure period ended, so we were unable to adjust for baseline levels of cognitive function or assess change over time. This could potentially induce bias if individuals with poorer baseline cognition were more likely to stay in higher-exposure jobs, incurring additional damage from solvents. While we are unable to test for this possibility, our JEM takes account of lifetime occupational position in calculating exposure, so we were able to indirectly control for occupational trajectory. We also adjusted for education, a known premorbid determinant of cognitive function, and for age. However, repeat assessments of cognitive function would have permitted analysis of both causal ordering and cognitive change over time. For a small number of participants, we have a measure of MMSE and DSST taken 6 years prior3,20; however, we were unable to examine change over time because of small cells created by stratified analyses.
Because the present study involved multiple statistical tests, certain findings—such as the different results for phonemic and semantic fluency—could be attributable to chance. The consistency of our results and their concordance with prior literature generally points to a nonspurious effect. Nevertheless, the many domains of cognitive function tested and the several quantifications of lifetime occupational solvent exposure performed mean that our analysis perhaps generates as many hypotheses as it tests.
Lastly, although we controlled for sociodemographic, psychological, physiologic, and behavioral factors that could contribute to cognitive deficits, there may be residual confounding by factors such as cardiovascular disease, diet, and physical activity related to both occupational exposures and cognitive status, which could partially explain the observed relationship. However, we were able to adjust for proxy variables such as hypertension and other health indicators (smoking and BMI) that tend to covary with diet and physical activity,35 increasing confidence that alternative explanations were not responsible for the observed effects.
The use of a prospective design and validated JEM eliminates risk of differential misclassification of exposure regarding the outcome, but nondifferential misclassification is possible. This is less of a risk for matrices such as MATEX that are constructed for use in a particular company or industry, and in any case, such misclassification would either flatten exposure-response curves or bias results toward the null.36 To test for selection bias into the sample, we compared solvent exposure statuses of eligible participants who declined examination. We found that the highly exposed were more likely to decline participation (e.g., for chlorinated solvents, 20% of nonparticipants were highly exposed vs 16% of participants). The resultant lower average exposure level among those in our sample could bias results toward the null.
A strength of our study is our exposure assessment tool that was independent of cognitive tests and provided quantitative estimates of exposure dose and timing. Workers were generally hired by EDF-GDF as young adults and remained there until retirement, permitting greater precision than population-based JEMs.21,37 Exposure assessments that are weak either because of data collection (e.g., self-reported and retrospective, which may be subject to recall bias with respect to current cognitive function) or data quality (e.g., dichotomous exposure metrics) have been identified as major weaknesses in the literature on solvent exposure and cognitive function; our study addresses both gaps.1,4,38
We find that occupational solvent exposure during working life predicts postretirement deficits in general cognitive status, psychomotor speed, immediate recall, semantic fluency, and executive function among retirees, particularly those with high lifetime exposure, recent exposure, or both. These findings strengthen evidence of detrimental effects of occupational solvents on workers' cognitive health and provide a more complete picture of long-term effects of solvent exposure on multiple domains of cognitive function in retirement. Particularly because solvent exposure levels in the French working population are ubiquitous (used in nearly all manual tasks), high (12%–13%), and not decreasing among current cohorts of working adults,39 such exposures remain a real risk to the present and future cognitive health of workers. This is of particular importance in the present context of rising retirement ages in industrialized countries. One of the main challenges in extending working lives is ensuring that workers maintain good physical, mental, and cognitive health as they age. Organizations, payers, and society may benefit from both a more productive workforce and a reduction in workers' postretirement health care costs by protecting them from adverse exposures. Clinically, retired patients who experienced prolonged exposure to solvents during working life may benefit from cognitively stimulating activities to offset damage. By asking retired patients about former occupational exposures, physicians may be able to target such efforts toward patients who could derive maximum benefit.
Supplementary Material
ACKNOWLEDGMENT
We gratefully acknowledge the contribution of the participating Health Screening Centers.
GLOSSARY
- BMI
body mass index
- CI
confidence interval
- DSST
Digit Symbol Substitution Test
- EDF-GDF
Electricite de France–Gaz de France
- FCSRT
Free and Cued Selective Reminding Test
- JEM
job exposure matrix
- MMSE
Mini-Mental State Examination
- TMT
Trail Making Test
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Sabbath coformulated hypotheses, coguided analyses, and drafted the manuscript. Ms. Gutierrez performed all statistical analyses in collaboration with Drs. Sabbath and Berr. Dr. Okechukwu edited the manuscript. Drs. Singh-Manoux and Amieva edited the manuscript and provided expertise on the neuropsychological assessment instruments. Drs. Goldberg and Zins codirect the GAZEL cohort and edited the manuscript. Dr. Berr oversaw the cognitive data collection, coformulated hypotheses, oversaw analyses, and edited the manuscript.
STUDY FUNDING
This work is part of a project funded by the Agence Nationale de la Recherche (ANR, French National Research Agency), and Agence Française de Sécurité Sanitaire de l'Environnement et du Travail (AFSSET, French Agency for Sanitary Security of Environment and Work). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURE
E. Sabbath, L. Gutierrez, and C. Okechukwu report no disclosures relevant to the manuscript. A. Singh-Manoux receives research support from the NIH (NIA R01AG013196 [PI], R01AG034454 [PI]). H. Amieva reports no disclosures relevant to the manuscript. M. Goldberg received a grant from the Agence Nationale de la Recherche (WORKAGE ANR-08-BLAN-0028-01). M. Zins received a grant from the Agence Nationale de la Recherche (WORKAGE ANR-08-BLAN-0028-01). C. Berr received a grant from the Agence Nationale de la Recherche (WORKAGE ANR-08-BLAN-0028-01). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Dick FD. Solvent neurotoxicity. Occup Environ Med 2006;63:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker EL. Organic solvent neurotoxicity. Annu Rev Public Health 1988;9:223–232 [DOI] [PubMed] [Google Scholar]
- 3.Berr C, Vercambre MN, Bonenfant S, Singh Manoux A, Zins M, Goldberg M. Occupational exposure to solvents and cognitive performance in the GAZEL cohort: preliminary results. Dement Geriatr Cogn Disord 2010;30:12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer-Baron M, Blaszkewicz M, Henke H, et al. The impact of solvent mixtures on neurobehavioral performance: conclusions from epidemiological data. Neurotoxicology 2008;29:349–360 [DOI] [PubMed] [Google Scholar]
- 5.Dick FD, Bourne VJ, Semple SE, et al. Solvent exposure and cognitive ability at age 67: a follow-up study of the 1947 Scottish Mental Survey. Occup Environ Med 2010;67:401–407 [DOI] [PubMed] [Google Scholar]
- 6.van Valen E, Wekking E, van der Laan G, Sprangers M, van Dijk F. The course of chronic solvent induced encephalopathy: a systematic review. Neurotoxicology 2009;30:1172–1186 [DOI] [PubMed] [Google Scholar]
- 7.Kukull WA, Larson EB, Bowen JD, et al. Solvent exposure as a risk factor for Alzheimer's disease: a case-control study. Am J Epidemiol 1995;141:1059–1071 [DOI] [PubMed] [Google Scholar]
- 8.Daniell WE, Claypoole KH, Checkoway H, et al. Neuropsychological function in retired workers with previous long-term occupational exposure to solvents. Occup Environ Med 1999;56:93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordling Nilson L, Karlson B, Nise G, Malmberg B, Ørbæk P. Delayed manifestations of CNS effects in formerly exposed printers: a 20-year follow-up. Neurotoxicol Teratol 2010;32:620–626 [DOI] [PubMed] [Google Scholar]
- 10.Singh-Manoux A, Kivimaki M, Glymour MM, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ 2012;344:d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg M, Leclerc A, Bonenfant S, et al. Cohort profile: the GAZEL cohort study. Int J Epidemiol 2007;36:32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zins M, Bonenfant S, Carton M, et al. The CONSTANCES cohort: an open epidemiological laboratory. BMC Public Health 2010;10:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 14.Hoyer WJ, Stawski RS, Wasylyshyn C, Verhaeghen P. Adult age and digit symbol substitution performance: a meta-analysis. Psychol Aging 2004;19:211–214 [DOI] [PubMed] [Google Scholar]
- 15.Wechsler D. Wechsler Adult Intelligence Scale–Revised. New York: The Psychological Corporation; 1981 [Google Scholar]
- 16.Grober E, Buschke H. Genuine memory deficits in dementia. Dev Neuropsychol 1987;3:13–36 [Google Scholar]
- 17.Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia 1967;5:135–140 [Google Scholar]
- 18.Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol 2000;22:518–528 [DOI] [PubMed] [Google Scholar]
- 19.Tombaugh TN. Trail making test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 2004;19:203–214 [DOI] [PubMed] [Google Scholar]
- 20.Sabbath E, Glymour M, Berr C, et al. Occupational solvent exposure and cognition: does the association vary by level of education? Neurology 2012;78:1754–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg M, Imbernon E. The use of job exposure matrices for cancer epidemiology research and surveillance. Arch Public Health 2002;20:173–185 [Google Scholar]
- 22.Imbernon E, Goldberg M, Guénel P, et al. Matex: une matrice emplois-expositions destinée à la surveillance épidémiologique des travailleurs d'une grande entreprise (EDF-GDF). Arch Mal Prof 1991;52:559–566 [Google Scholar]
- 23.Goldberg M, Chevalier A, Imbernon E, Coing F, Pons H. The epidemiological information system of the French National Electricity and Gas Company: the SI-EPI Project. Med Lav 1996;87:16–28 [PubMed] [Google Scholar]
- 24.Barberger-Gateau P, Chaslerie A, Dartigues JF, Commenges D, Gagnon M, Salamon R. Health measures correlates in a French elderly community population: the PAQUID Study. J Gerontol 1992;47:S88–S95 [DOI] [PubMed] [Google Scholar]
- 25.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist 1970;10:20–30 [DOI] [PubMed] [Google Scholar]
- 26.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 27.Nilson LN, Barregård L, Sällsten G, Hagberg S. Self-reported symptoms and their effects on cognitive functioning in workers with past exposure to solvent-based glues: an 18-year follow-up. Int Arch Occup Environ Health 2007;81:69–79 [DOI] [PubMed] [Google Scholar]
- 28.Nilson LN, Sällsten G, Hagberg S, Bäckman L, Barregård L. Influence of solvent exposure and aging on cognitive functioning: an 18 year follow up of formerly exposed floor layers and their controls. Occup Environ Med 2002;59:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li CY, Sung FC. A review of the healthy worker effect in occupational epidemiology. Occup Med 1999;49:225–229 [DOI] [PubMed] [Google Scholar]
- 30.Juntunen J. Neurotoxic syndromes and occupational exposure to solvents. Environ Res 1993;60:98–111 [DOI] [PubMed] [Google Scholar]
- 31.Grogan A, Green DW, Ali N, Crinion JT, Price CJ. Structural correlates of semantic and phonemic fluency ability in first and second languages. Cereb Cortex 2009;19:2690–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weuve J, Korrick SA, Weisskopf MA, et al. Cumulative exposure to lead in relation to cognitive function in older women. Environ Health Perspect 2009;117:574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang CY, Carpenter DM, Eaves EL, et al. Occupational solvent exposure and brain function: an fMRI study. Environ Health Perspect 2011;119:908–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raz N, Gunning FM, Head D, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex 1997;7:268–282 [DOI] [PubMed] [Google Scholar]
- 35.Fine LJ, Philogene GS, Gramling R, Coups EJ, Sinha S. Prevalence of multiple chronic disease risk factors: 2001 National Health Interview Survey. Am J Prev Med 2004;27:18–24 [DOI] [PubMed] [Google Scholar]
- 36.Martin JC, Imbernon E, Goldberg M, Chevalier A, Bonenfant S. Occupational risk factors for lung cancer in the French Electricity and Gas Industry. Am J Epidemiol 2000;151:902–912 [DOI] [PubMed] [Google Scholar]
- 37.Goldberg M, Kromhout H, Guenel P, et al. Job exposure matrices in industry. Int J Epidemiol 1993;22:S10–S15 [DOI] [PubMed] [Google Scholar]
- 38.Gun RT, Korten A, Jorm A, et al. Occupational risk factors for Alzheimer disease: a case-control study. Alzheimer Dis Assoc Disord 1997;11:21–27 [DOI] [PubMed] [Google Scholar]
- 39.Arnaudo B, Léonard M, Sandret N, Cavet M, Coutrot T, Rivalin R. L'évolution des risques professionnels dans le secteur privé entre 1994 et 2010. Dares Analyses 2012 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


