Figure 7.
Modularity of Network Masks Critical Roles for Su(dx) and Dx during Drosophila Embryogenesis
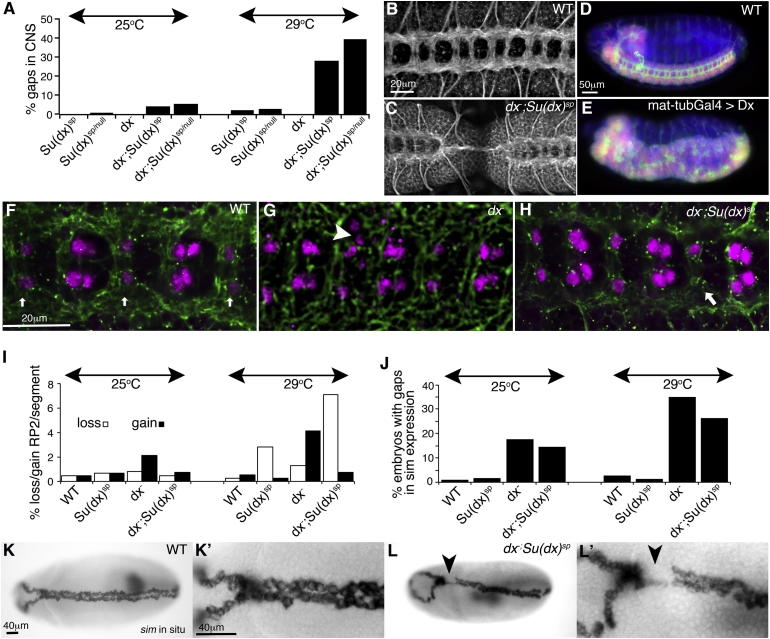
(A) dx;Su(dx) maternal/zygotic mutant embryos show more frequent and temperature-dependent gaps in the central nervous system (CNS) compared to Su(dx) or dx (p < 0.005, n > 30).
(B) Anti-Hrp-stained CNS of WT stage 15-16 embryo.
(C) CNS loss in dx;Su(dx) embryo.
(D) WT stage 15 embryo CNS, anti-ELAV (red), anti-HRP (green), DAPI (blue).
(E) Neurogenic phenotype after Dx expression using mat-tubGal4.
(F) WT embryo, anti-Eve (purple), anti-Hrp (green). Pairs of RP2 neurons are indicated by arrows.
(G) Extra RP2 neurons in dx (arrowhead).
(H) Loss of RP2 in dx;Su(dx) embryo (arrow).
(I) RP2 loss in dx;Su(dx) at 29°C is more frequent than for either mutant on its own or for dx;Su(dx) at 25°C (p < 0.01). A gain of RP2s was observed in dx compared to WT at 29°C, p < 0.01 (>230 segments per genotype scored at stage 15/16).
(J) Reduced sim expression in stage 7-8 dx embryos, (p < 0.001, n > 60), with increased penetrance at higher temperature. The dx phenotype was not strongly reduced by Su(dx).
(K–L′) In situ staining of sim in WT (K) and dx;Su(dx) (L) embryos. (K′) and (L′) show enlarged images of similar areas of (K) and (L) where arrowheads indicate gap in sim expression in (L) and (L′). Statistics by Fisher’s exact test.