Figure 5.
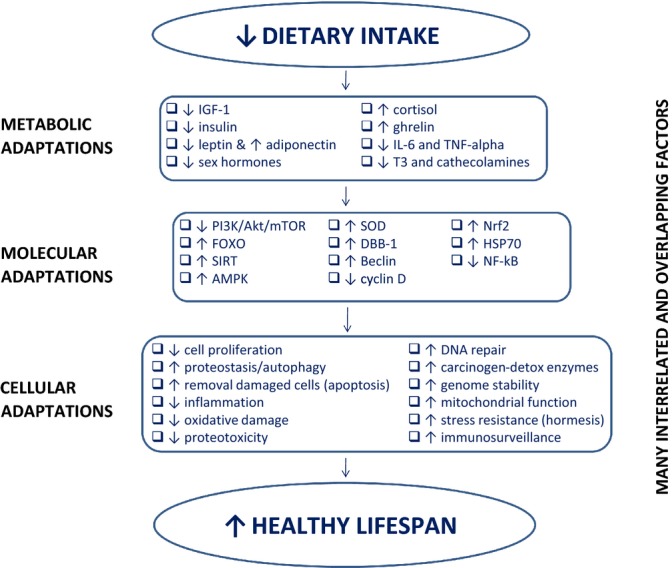
Dietary restriction (DR)-induced metabolic, molecular, and cellular adaptations. A chronic negative energy balance induced by dietary restriction triggers multiple metabolic adaptations including a reduction in circulating levels of growth factors (i.e., IGF-1), anabolic hormones (e.g., insulin, testosterone, estradiol, leptin), inflammatory cytokines (e.g., IL-6 and TNF-alpha), and key hormones that regulate thermogenesis and cellular metabolism (e.g., triiodothyronine (T3), cathecolamines), and an increase in hormones that suppress inflammation (e.g., cortisol, ghrelin, and adiponectin). Concomitantly, several key molecular adaptations take place, including a down-regulation of the insulin/IGF pathway (i.e., PI3K/Akt/mTOR), and an upregulation of two energy-sensing pathways (i.e., SIRT and AMPK) which activates FOXO. The activation of FOXO in turn modifies several ‘longevity genes,’ including up-regulation of antioxidant genes (e.g., SOD2), DNA repair genes (e.g., DDB1), autophagy genes (e.g., beclin), and a down-regulation of genes that control cell proliferation (e.g., cyclin D). DR induces also a down-regulation of inflammatory pathways (e.g., NFkB), and an up-regulation of genes that enhance protection against molecular damage (e.g., Nrf2, HSP70). These DR-induced metabolic and molecular modifications are responsible for several important cellular adaptations, that antagonize the accumulation of macromolecular damage, abnormal cell proliferation, and cellular senescence (Martin et al., 2006; Fontana et al., 2007a,b; Fontana et al., 2010; Guarente, 2013; Selman & Withers, 2011; Chen et al., 2010).
