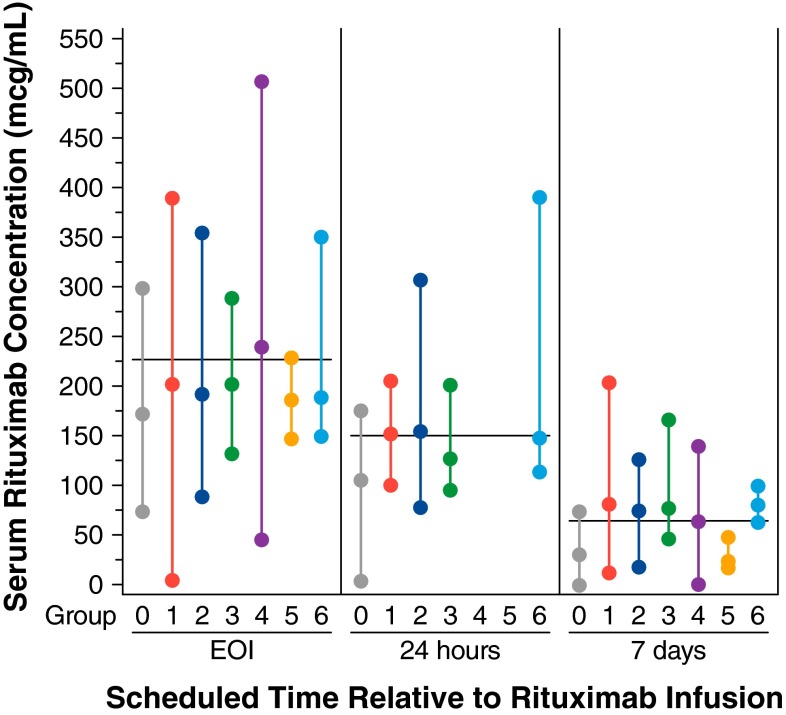
Fig. 3.
Median rituximab concentrations (min, max) over time from the bendamustine–rituximab combination study in the presence or absence of bendamustine. The lowest, middle, and highest symbols represent the minimum, median, and maximum of the observed rituximab concentrations, respectively. The horizontal line represents the weighted average of the median concentrations reported in literature only. Note For the bendamustine–rituximab combination study (Group 0), EOI concentrations were collected prior to bendamustine administration. The 24-h and 7-day concentrations were collected following bendamustine administration. For all other groups, rituximab was administered in the absence of bendamustine. Group 0: the bendamustine–rituximab combination study; n = 19; advanced indolent NHL or MCL. Group 1: NHL with low tumor burden [29]. Group 2: n = 14; autoimmune disorders [29]. Group 3: n = 4; amyloid light-chain amyloidosis [29]. Group 4: n = 137; recurrent low-grade or follicular NHL [30]. Group 5: n = 10; advanced follicular lymphoma and MCL [31]. Group 6: n = 7; follicular lymphoma [32]. EOI end of infusion, MCL mantle cell lymphoma, NHL non-Hodgkin lymphoma