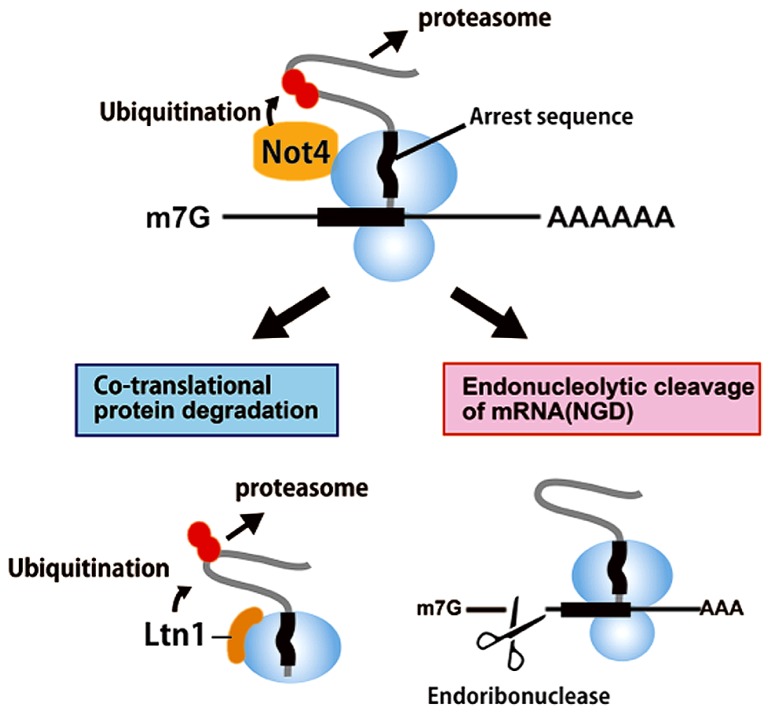
FIGURE 3.
A novel role of CCR4-NOT in protein quality control by translation arrest.The NOT4 subunit of the yeast CCR4-NOT complex is an E3 ubiquitin ligase. EGD, an ortholog of the nascent associated polypeptide complex (NAC), is the first described substrate of NOT4 in yeast. In addition of Ltn1, NOT4 is involved in co-translational clearance of aberrant proteins by the proteasome in yeast. The stalling of ribosomes during translational elongation results in co-translational degradation of the arrested protein product by the proteasome (ribosome quality control, RQC) as well as an endonucleolytic cleavage of mRNA no-go decay (NGD). NOT4 synergizes with the important E3 ligase Ltn1 for the degradation of arrest products. Ltn1 has been suggested to recognize the 60S subunit containing peptidyl-tRNA, which arises from the dissociation of the ribosome into 40S and 60S subunits by an unknown mechanism.