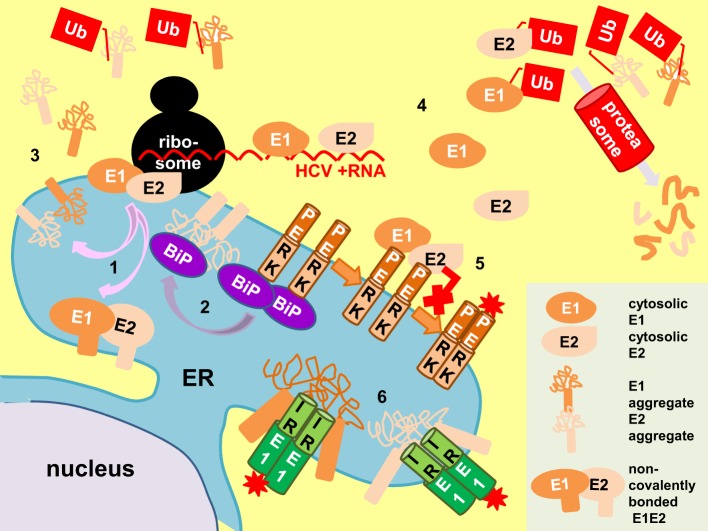
Figure 5.
Proposed mechanisms of E1/E2 activation of UPR. E1 and E2 are targeted and mature in the endoplasmic reticulum (ER) to form non-covalently-bonded heterodimers and disulphide-bonded aggregates (pink arrows, 1). The E2 aggregates distract BiP from PERK (purple arrow), allowing PERK oligomerization and activation (brown arrows, 2). Retrotranslocated (3) and cytosolic E1/E2 from surplus protein synthesis (4) can also activate the unfolded protein response (UPR) by perturbation of proteasomal function (Ub, polyubiquitin). Conversely, cytosolic E1/E2 can bind to the cytoplasmic domain of PERK and inhibit its activation (red cross, 5). Direct binding of E1 and E2 aggregates to the luminal domain of IRE1 can also activate UPR (6). Red asterisk, activation by phosphorylation.