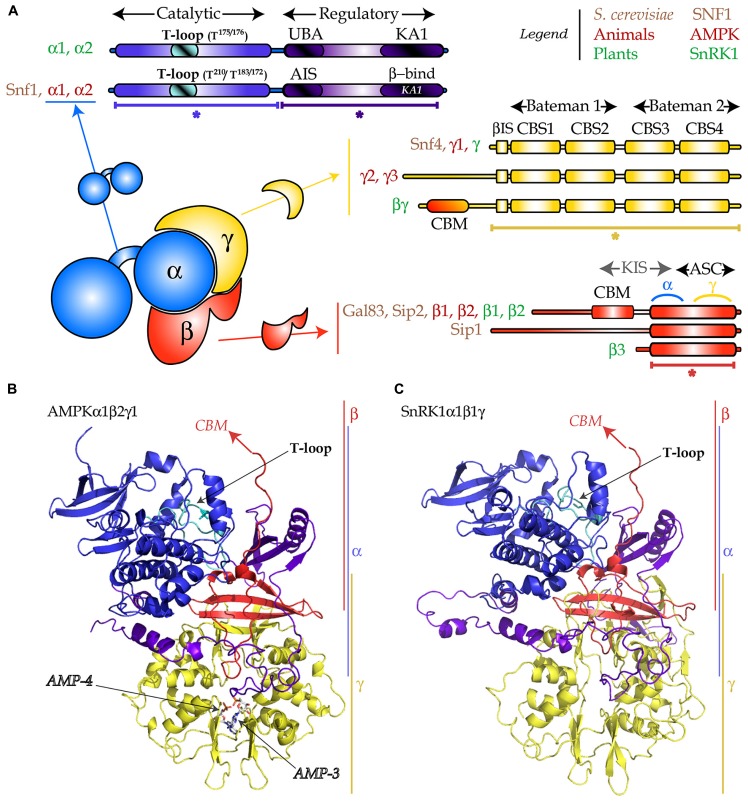
FIGURE 1.
Heterotrimeric structure of the SNF1/AMPK/SnRK1 complexes. (A) The α-subunit (in blue) is composed of a catalytic domain (in blue with the T-loop in cyan) and a regulatory domain (in purple-blue) which encompasses an auto-inhibitory sequence (AIS) or an ubiquitin-associated (UBA) domain, and a kinase-associated (KA1) domain for binding the β- and γ-subunits. The γ-subunit (in yellow) is composed of two “Bateman” domains each of them containing two CBS (cystathionine-β-synthase) domains and a β-interacting sequence (βIS). The AMPKγ2 and γ3 bear an N-terminal extension and the plant-specific SnRK1βγ possesses a carbohydrate binding module (CBM). The β-subunit (in red) harbors an ASC (association to the complex) domain, containing the sites of interaction with γ and α, a CBM and an N-terminal extension. The KIS (kinase interacting sequence) domain, traditionally used for designating the region comprising the CBM and the site for interaction with the α-subunit, is no longer used. The plant-specific SnRK1β3 is atypical as it does not possess the CBM or the N-terminal extension. (B) Cartoon representation of the 3D-structure (PDB: 2Y94) of the AMPKα1β2γ1 complex. Asterisks designate parts in (A) that were crystallized. Arrows indicate missing parts (CBM), the T-loop, and the two AMP molecules. (C) 3D-structure model of SnRK1α1β1γ, generated with Swiss-Model using as template the AMPK structure presented in (B). Color code in (B,C) as described in (A).