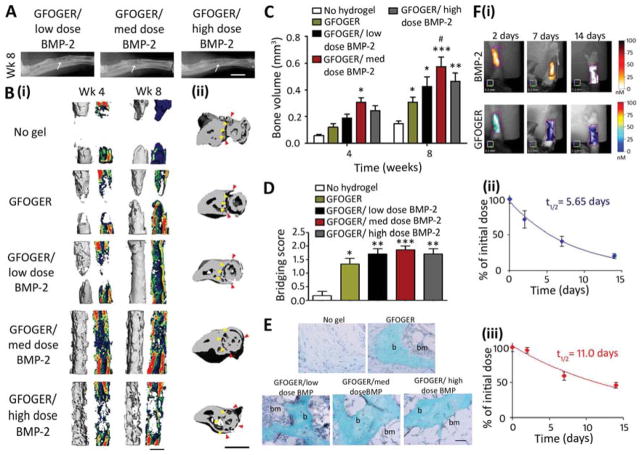
Fig. 3.
GFOGER-functionalized PEG hydrogels with low-dose BMP-2 bridge radial segmental defects without altering ulnar structure. (A) Radiographic images, white arrows indicate space between ulna and radius which is not present in the high BMP-2 dose image, scale bar 2 mm. (B) 3D μCT reconstructions of (i) radius in sagittal view (left) with mineral density mapping (right), and (ii) radius and ulna in transverse view. Yellow arrowheads indicate boundary between the ulna and radius prior to implantation, red arrowheads indicate the position of the ulna closest to the radius at 8 weeks, scale bar 1 mm. (C) μCT measures of bone formation, n=6–7. (D) Scoring of defect bridging at 8 weeks, n=6–7. (E) Sections stained with Safranin O/Fast Green at the center of defect, scale bar 50 μm. b - bone, bm - bone marrow. (F) (i) Representative FMT images and FMT quantification of % implanted dose retained in radial defect space over time in vivo for (ii) high dose BMP-2 labeled with Vivotag 800 and (iii) GFOGER peptide labeled with Vivotag 680, n=6. * p<0.05, **p<0.01, ***p<0.001 compared to defect receiving no hydrogel implant, # p<0.05 compared to GFOGER hydrogel.