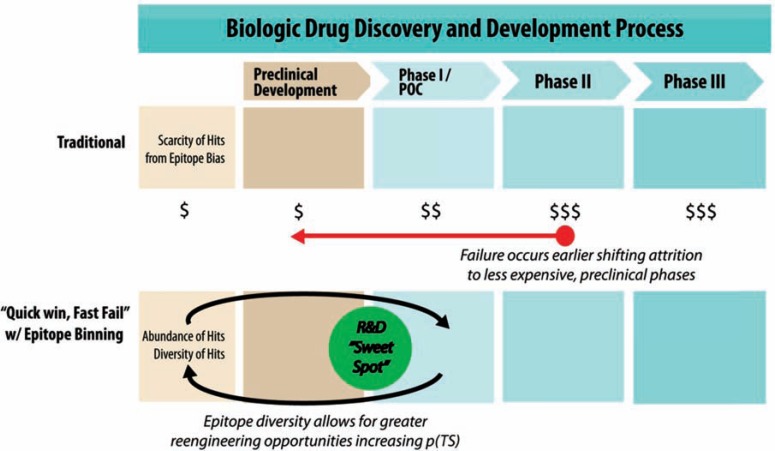
Fig. (3).
Graphic illustrating the traditional drug development process versus a process with epitope binning referred to as “quick win, fast fail”. In the epitope binning process, failures and technical uncertainty are decreased earlier in the process before expensive Phase II and Phase III trials. This results in a broader more well characterized number of biologics advancing into Phase II and III and those that do advance have a higher probability of success p(TS) and launch (adapted from Paul et al.) [8]. Between preclinical development and phase I/ proof-of-concept phase is the R&D “sweet spot.”