Table 1. Selected Preliminary Experiments.
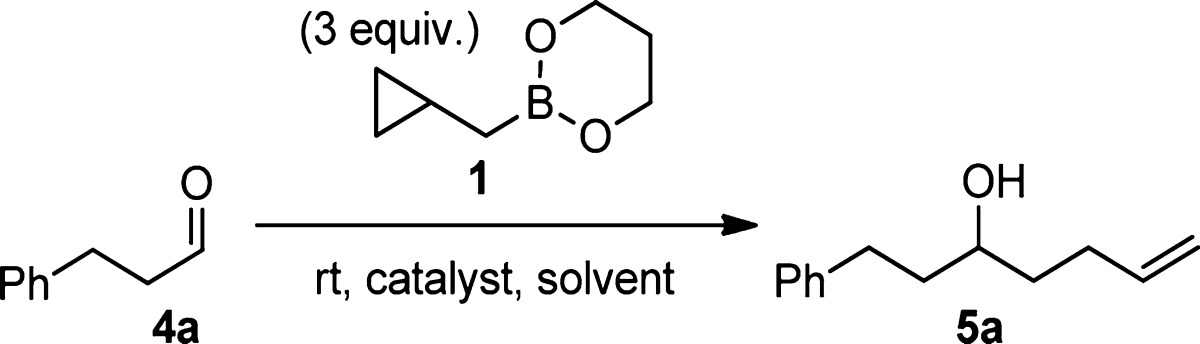
| entry | catalyst | amount (mol %) | solvent | time (h) | yielda (%) |
|---|---|---|---|---|---|
| 1 | PhBCl2 | 15 | DCM | 3 | 0b |
| 2 | Sc(OTf)3 | 5 | CDCl3 | 2 | 0b |
| 3 | Sc(OTf)3 | 10 | CDCl3 | 20 | 0b |
| 4 | AlCl3 | 10 | CDCl3 | 24 | 0b |
| 5 | Cu(OTf)2 | 10 | CDCl3 | 168 | 0b |
| 6 | B(OTFA)3 | 15 | DCM | 0.5 | 74c |
| 7 | B(OTFA)3 | 15 | Et2O | 18 | 3 |
| 8 | B(OTFA)3 | 15 | THF | 18 | 1 |
| 9 | B(OTFA)3 | 15 | pentane | 4 | 1 |
| 10 | B(OTFA)3 | 15 | toluene | 1.5 | 26 |
NMR yield, except entry 6.
Forcing the reaction with higher temperatures resulted in self-aldol products from 4a and/or ring opening of reagent 1.
Isolated yield.
