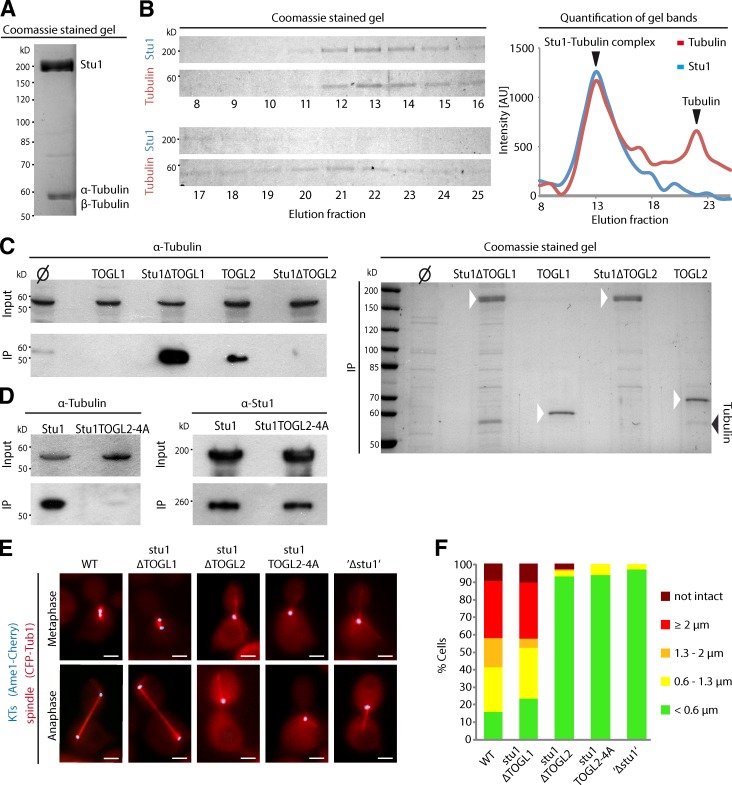
Figure 2.
TOGL2, but not TOGL1, binds tubulin and is essential for spindle formation. (A) Tubulin copurifies with Stu1. FLAG-Stu1-GFP was affinity purified (see Materials and methods). Copurified tubulin was identified by mass spectrometry. (B) Stu1 and tubulin coelute as a complex during gel filtration. Fractions were analyzed as indicated and quantified. The data shown are from a single representative experiment out of two analyses. AU, arbitrary unit. (C) TOGL2, but not TOGL1, mediates tubulin binding. The constructs were expressed and purified as in A. Similar amounts of the IPs were loaded and subjected to Western analyses (α-tubulin) or PAGE/Coomassie. Ø indicates that no FLAG-tagged Stu1 construct was expressed. White arrowheads indicate the Stu1 constructs. (D) Mutations in the intra-HEAT repeat loops of TOGL2 abolish the Stu1–tubulin interaction. Constructs were expressed and purified as in A. (E) Cells were released from G1 arrest and visualized in meta- and anaphase. To analyze stu1ΔTOGL2, stu1TOGL2-4A, and ‘Δstu1’ cells, WT Stu1 expressed in the background was depleted. Bars, 2 µm. (F) Spindle phenotypes were quantified as indicated 2 or 2.5 h (Fig. S2) after G1 release. n > 100.