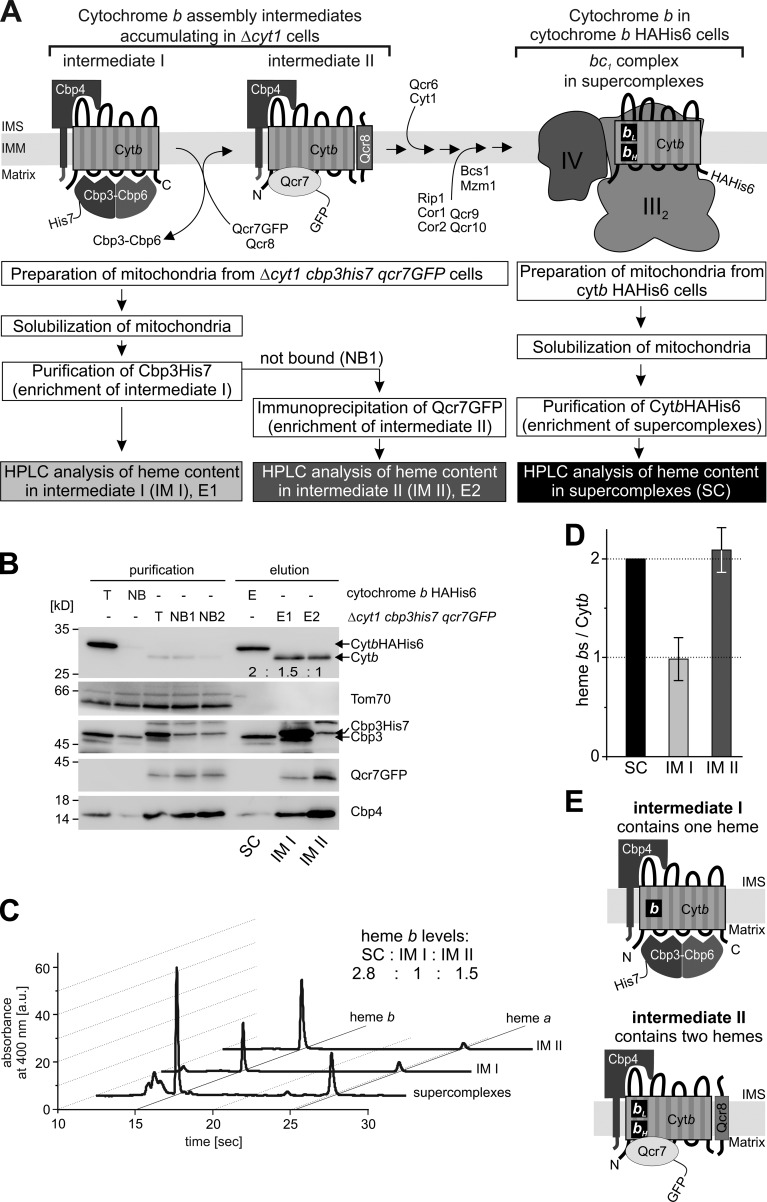
Figure 2.
Cytochrome b is semi-hemylated in assembly intermediate I but fully hemylated in assembly intermediate II. (A) Schematic representation of the experimental setup. To accumulate and specifically purify intermediate I or II from the same sample, a yeast strain with a cytochrome c1 deletion was constructed that carries both a His7-tagged Cbp3 and a GFP-tagged Qcr7. Mitochondria isolated from this stain were lysed in 1% digitonin. After intermediate I was enriched (E1) by affinity purification of Cbp3-His7, the not-bound fraction (NB1) was recovered and Qcr7-GFP was purified by immunoprecipitation using a GFP nanobody (E2). The fully assembled bc1 complex was purified from mitochondria carrying the cytochrome b HAHis6 variant (E). All eluates were split and analyzed by both Western blotting (B) with the antibodies indicated and HPLC (C) after heme extraction. The numbers in the Western blot of Cytb denote the relative cytochrome b levels that were densitometrically determined. (D) Relative heme b/cytochrome b protein ratios were calculated and the values for heme extracted from supercomplexes were set to 100% (reflecting two heme bs/cytochrome b protein). Mean values of four independent experiments ± SEM are shown. (E) Graphical summary, showing fully hemylated cytochrome b in intermediate II and a semi-hemylated cytochrome b in intermediate I. IM I, intermediate I; IM II, intermediate II; SC, supercomplexes.