Significance
The CRE/LoxP cell lineage tracing strategy has been applied effectively to label progenies derived from specific progenitors in different model organisms. Although this approach efficaciously labels cells expressing a specific marker, it often discounts the heterogeneity of the cell populations sharing the same cellular marker. In this study, we combined the CRE/LoxP tracing strategy with BrdU birth-dating analysis to separate the NG2 expression progenitor populations and identified the defined interneuronal versus oligodendroglial lineages on the basis of special and temporal specific origins.
Keywords: lineage differentiation
Abstract
The studies on the exact lineage composition of NG2 expressing progenitors in the forebrain have been controversial. A number of studies have revealed the heterogeneous nature of postnatal NG2 cells. However, NG2 cells found in embryonic dates are far less understood. Our study indicates that early NG2 progenitors from a ventral origin (i.e., before embryonic day 16.5) tangentially migrate out of the medial ganglionic eminence and give rise to interneurons in deep layers of the dorsal cerebral cortex. The majority of myelinating oligodendrocytes found in both cortical gray and white matters are, in contrast, derived from NG2 progenitors with a neonatal subventricular zone origin. Our lineage tracing data reflect the heterogeneous nature of NG2 progenitor populations and define the relationship between lineage divergence and spatiotemporal origins. Beyond the typical lineage tracing studies of NG2+ cells, by costaining with lineage-specific markers, our study addresses the origins of heterogeneity and its implications in the differentiation potentials of NG2+ progenitors.
The relationship between progenitor origins and their possible terminal cell fates in the central nervous system (CNS) development is a complex question that remains to be fully addressed. Depending on the origin from which a progenitor cell arises, physical and molecular regulatory mechanisms define both cell identity and direct specific lineage potentials during differentiation and migration. For example, cortical pyramidal neurons are generated in the ventricular zone (VZ) of the pallium and are guided by radial glia to their final position in the cortical plate (1, 2). However, cortical interneurons born in subpallium germinal zones during early embryonic dates tangentially migrate to the cortical plate up to the neonatal period. Not only neurons but also the differentiation of glial cells follow a specific spatial and temporal patterning (3, 4). The developmental origin of oligodendrocytes (OLs) is a longstanding controversial issue with many valid hypotheses (5). One hypothesis suggests that OLs are developed throughout all regions of the CNS, with multiple and diverse developmental origins that provide the progenitor sources of all OLs (6, 7). This hypothesis was challenged in the early 1990s as a series of observations suggested that commitment to the OL lineage occurs in a specialized domain of the ventral VZ in development of the spinal cord and forebrain (8). Both strategies provide mature OLs for myelination, but separate and distinct developmental regulatory strategies that direct the OL development are required for each scenario.
The subpallium germinal zones are divided into three areas: the medial ganglionic eminence (MGE), the lateral ganglionic eminence, and the caudal ganglionic eminence. The distribution of cortical interneurons correlates with the origin of their progenitors (9, 10). Additional genetic studies have demonstrated that NG2 cells in the subpallium give rise to cortical interneurons (11). However, it remains unresolved whether cortical interneurons and OLs share the same progenitor pool. Collectively, the aforementioned findings underscore the need for reconciling the differentiation potential of heterogeneous NG2+ progenitors. Our genetic lineage tracing, together with BrdU pulse-labeling, identified two independent origins of NG2 progenitors in the forebrain. The first group is MGE-derived NG2+ progenitor cells that are born at early embryonic dates and give rise to deep-layer interneurons in dorsal cortex (CTX). The second NG2+ progenitor pools are found in the postnatal subventricular zone (SVZ), derived from GFAP+ neural stem/progenitor cells (NPCs). This particular progenitor pool includes the classical OPCs, responsible for generating mature OLs found in corpus callosum (CC) and dorsal CTX.
Results
NG2-Cre BAC Transgenic Mice as a Model System.
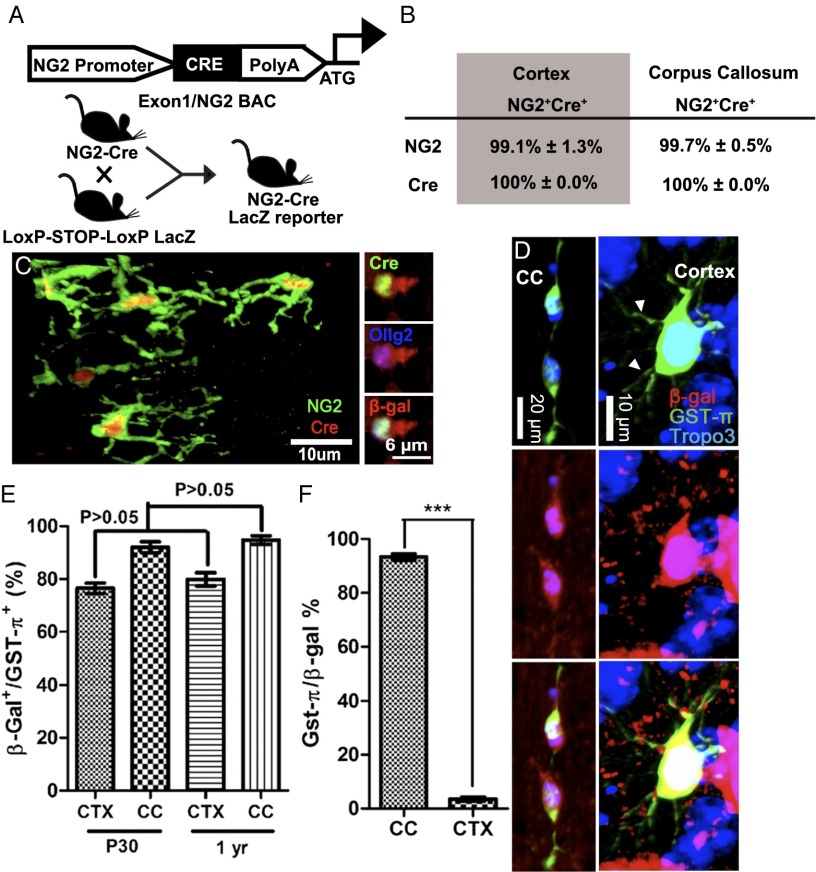
The NG2+;PDGFαR+ cells in the CNS are characterized as OPCs that give rise to mature OLs in both white and gray matter. Recent evidence suggests that CNS NG2+;PDGFαR+ OPCs are more diverse in terms of their electrophysiological responses and possible lineage potentials (12–14). In general, studies on the lineage potential of NG2+;PDGFαR+ cells have focused on postnatal dates. Several findings have indicated that the peak of oligodendrogliogenesis occurs during early postnatal dates and then slows down but remains continuous at low levels in both white and gray matter in the adult CNS. All these studies provided strong evidence for the important biological function of postnatal NG2+/PDGFαR+ progenitors in the mammalian CNS. However, NG2+/PDGFαR+ progenitors can also be found within early embryonic CNS [embryonic day (E)14–E18] at basal ganglia (5, 15). These embryonic NG2+;PDGFαR+ progenitors are assumed to be the progenitors that eventually differentiate into mature OLs in the adult CNS. This raised the question of whether these diverse NG2 cell populations give rise exclusively to OLs in the CNS or whether different terminal cell fates are possible. To determine whether all NG2+ precursors found in CC and dorsal CTX are single-lineage, we traced cells using genetic fate mapping by crossing the Ng2-Cre mice that we generated with the Cre-LoxP reporter mice, Rosa26-LacZ (Fig. 1A). The Cre recombinase activity under the control of CSPG4 (the gene encoding the NG2 protein) promoter regulation would induce permanent and heritable expression of the reporter gene β-galactosidase (β-gal) through mitosis. To confirm the correlation of Cre-expressing cells and NG2+ cells, we focused on the costaining analysis throughout developmental stages, starting at embryonic dates to postnatal day 30 (P30), and showed that close to 100% of NG2+ cells were also Cre+ and vice versa in both CC and CTX (Fig. 1A). From the costaining of Cre and β-gal, the recombination efficiency of Cre regulated by CSPG4 promoter was found to be about 98% (Fig. 1 B and C).
Fig. 1.
Schematic representation and validation of transgenically targeted fate mapping strategy. (A) The Cre recombinase transgene was inserted into the first exon of the NG2 (CSPG4) promoter, and Ng2-Cre mice were crossed with reporter Rosa26LacZ mice. (B) Quantification of NG2+ and Cre+ cells found in CTX and CC through P0–P30 showed no regional difference of the correlation. (C) Double staining of Cre and NG2 showed the high correlation of Cre and NG2 expressions in a double-transgenic mouse line. All of the Cre+ cells also coexpress Olig2. (D) In the CC and CTX at age 1 y, the β-gal-labeled NG2-derived progenies costained with GST-π (arrowhead). (E) To estimate the percentage of OLs generated during adult oligodendrogliogenesis, GST-π+;β-gal+ cells were counted against GST-π+ cells. There was no significant change (P > 0.3) in NG2-derived OL percentages between these ages. (F) Comparing the NG2 progenitor-derived OLs in adult CC and CTX, only 3.52% ± 1.38% of NG2 progenies in CTX are labeled as GST-π+.
NG2 Progenitors Give Rise to OLs in Both CC and Dorsal CTX.
To inspect NG2 lineage development, we first examined whether NG2 progenitor cells that came from different developmental ages and origins all gave rise equally and exclusively to mature OLs in postnatal mice, as NG2 progenitors are historically considered to be exclusively OPCs (16). We costained the NG2 progenies with β-gal, along with mature OL markers, myelin basic protein and GST-π (Fig. S1F). Because GST-π stains the cell body, we analyzed NG2-derived OLs by counting GST-π+ cells (OL marker) within the β-gal+ population (total NG2-derived cells) in the dorsal CTX and CC regions at different postnatal ages. In P30 mouse CC, 92% ± 4.18% of all GST-π+ cells were β-gal+ (Fig. 1 D and E), and 93.3% ± 2.08% of all β-gal+ cells were GST-π+ terminally differentiated OLs (Fig. 1F). These percentages remained the same in 1-y-old mice (91.3% ± 3.025% of GST-π+ cells were β-gal+; Fig. 1E), suggesting that NG2+ progenitors in this region mainly undergo oligodendrogliogenesis. In the dorsal CTX at 1 y, ∼79.9% ± 4.97% of all GST-π+ cells were β-gal-labeled (n = 3) (Fig. 1 D and E). At P30, 76.5% of GST-π+ OLs were already colabeled with β-gal in dorsal CTX (Fig. 1E). These observations suggest that the majority of differentiated OLs located in the cortical gray matter are derived from NG2 progenitors, leaving only ∼20% of all myelinating OLs in the dorsal CTX derived from a non-NG2 progenitor source (Fig. 1E). In contrast, only 3.52% ± 1.38% of all β-gal+ cells (progeny derived from NG2+ cells) in the dorsal CTX became GST-π+ OLs, a stark difference from CC, where most (93.3%; Fig. 1F) NG2-derived progeny became OLs. Therefore, the vast majority of OLs in the CC and dorsal CTX are derived from NG2 progenitors, whereas only a very small proportion of NG2 progenitors in dorsal CTX were designated to an OL lineage, leaving it possible for cortical NG2 progenitors to take on other neural cell fates. In summary, our lineage tracing in CC is consistent with previous models suggesting that NG2+ cells are limited to an OL lineage. However, data from the dorsal CTX suggested that these models may not be true in all cases: Not all NG2 progenitors must become OLs. Therefore, NG2 progenitors may be able to differentiate into non-GST-π+ cells, depending on the region they originated, which suggests that not all NG2+ cells are OPCs. To address this, we looked at region-specific differences in the OPC-defining properties of NG2+ cells.
NG2 Lineage Development Shows Different Patterns in Early Postnatal Dorsal CTX and CC.
In postnatal CNS, oligodendrogliogenesis is a continuous process that requires the self-renewal activity of progenitors, namely, OPCs, in the form of active mitosis (17, 18). However, in our analyses, most NG2+ cells in the dorsal CTX did not give rise to OLs, and thus did not qualify as classical OPCs. If not all NG2+ progenitors are OPCs, then this might be reflected by differences in their mitotic properties. The quantification and morphology of NG2 progeny in the CC and dorsal CTX were analyzed at ages P0, P3, P5, P7, P15, and P30. Because NG2 immunostaining only outlines the cell membrane, we opted to count Cre+ cells as a reliable indicator of NG2+ cell numbers. Cells derived from NG2+ progenitors, or those still expressing NG2, were positive for β-gal. Using this quantification strategy, we observed that NG2+ (β-gal+;Cre+) cells within the CC increased substantially between P0 and P3 (from 4.2% ± 1.03% to 13.2% ± 1.37% of total cells). After P5, NG2+ cells gradually decreased in number and reached 5.4% ± 0.849% of total cells at P30. Unlike the CC, NG2+ cells found in dorsal CTX remained below 5% of total cells in gray matter from P0 to P30 (Fig. 2 A and B). Notably, at P30, some late postnatal NG2+ cells, albeit only a small number, were labeled with a proliferation marker, Ki67, suggesting that a fraction of NG2+ cells in both CC and dorsal CTX are still mitotically active in the postnatal brain (Fig. S1 A–D).
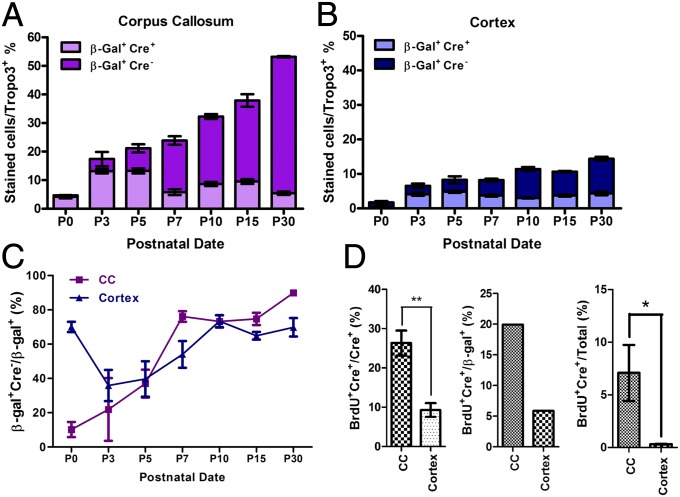
Fig. 2.
Proliferation of NG2 progenitor cells during postnatal development in CC vs dorsal CTX. (A) Within CC, nearly all of β-gal+ cells were NG2/Cre+ at P0. The percentage of β-gal+ cells showed a steady increase from P0 to P30. (B) At P0, β-gal+ cells found in dorsal CTX were mostly not NG2/Cre+ progenitors. At P3, a group of NG2/Cre+ cells entered the region. The peak of NG2/Cre+ cells was found at P5. (C) In P0 dorsal CTX, the majority of β-gal+ cells were differentiated progenies. At P3, a new wave of NG2+ progenitors entered the dorsal cortical region, which lowered the percentage of differentiating population in the total β-gal+ population. In CC, a continuous increase of differentiating β-gal cells was found from P0 to P30. (D) In vivo proliferation assay at P3 indicated that a significantly higher percentage of NG2 cells in CC are in mitotic state compared with dorsal CTX (P = 0.0095). Percentage of BrdU+;Cre+/Total (TO-PRO-3 staining, Invitrogen) showed that CC contains higher percentage of proliferative NG2 cells relative to dorsal CTX (P = 0.0485).
To estimate how many NG2-derived cells were still in a progenitor state, we compared β-gal+ with Cre+ cell numbers in CC, starting at P0, and found that 4.65% ± 1.21% of total cells were labeled with β-gal and that the vast majority of them (93%) were also Cre+, suggesting that most β-gal+ cells were not yet differentiated in CC at P0. However, we observed a surge in the β-gal+ population in CC after P0. At P3, 13.2% ± 1.34% of total cells in CC were β-gal and Cre (NG2+ progenitors) double-positive undifferentiated NG2 cells. Of total cells in that region, 4.24% ± 1.37% had lost NG2 expression, suggesting these cells were a terminally differentiated population. At P5, a higher percentage of β-gal+ cells had lost NG2 expression (Fig. 2 A and B), and after P7, the percentage of double labeled β-gal+;Cre+ cells decreased even further and remained low until P30. In contrast, a gradual but consistent expansion of β-gal+;Cre− (differentiated) progeny cells continued until P30. In summary, the expansion of NG2+ progenitors in CC peaked between P3 and P5, but terminal differentiation of progeny was detected from P3 until P30. Unlike in CC, the percentage of NG2+ cell population in dorsal CTX increased on a much smaller scale. At P0, although only a small number of cells could be detected, most were β-gal+ and Cre− in dorsal CTX, suggesting these cells were differentiating or had already differentiated. Most of these cells were found in layer 1 (Fig. S2E). The proportion of β-gal+ cells in dorsal CTX showed only a moderate increase from P0 to P30 compared with β-gal+ cells in CC (Fig. 2 A and B). By quantifying the number of β-gal+;Cre− cells relative to total β-gal+ cells (total NG2+ number plus progeny), we calculated the fraction of terminally differentiated NG2 progenies in CC and dorsal CTX at different postnatal dates (Fig. 2C).
Initially at P0, the majority (∼80%) of NG2-derived cells were already differentiated in dorsal CTX. However, at P3 we observed a drop in the percentage of differentiated NG2 progeny, indicating an emergence of undifferentiated NG2 cells in the CTX. In contrast, the majority of NG2-derived cells in CC were undifferentiated at P0 and differentiated gradually, following an expected time course. To evaluate the mitotic activity of NG2+ cells in CC and dorsal CTX, we labeled P3 Ng2-Cre/Rosa26LacZ mice with BrdU and compared BrdU-incorporated NG2+ populations between these two regions 3 h postlabeling. Among NG2+ cells, 26.3% ± 1.83% in CC were BrdU+ compared with 9.30% ± 1.01% in dorsal CTX (Fig. 2D). Moreover, among the whole β-gal+ population (including both Cre+ and Cre− populations), the amount of BrdU-labeled cells in CTX was only a quarter of that in CC (Fig. 2D). Thus, at P3, the emergence of an undifferentiated NG2 population (Cre+) is likely not only resulting from cell proliferation (as in the CC) but also from entrance of migratory postmitotic, yet not terminally differentiated, NG2 progenitor cells (Cre+). Therefore, lineage development of NG2+ progenitors was quite different in these two regions.
Neurogenesis and Oligodendrogliogenesis Start from Separate NG2 Progenitor Pools.
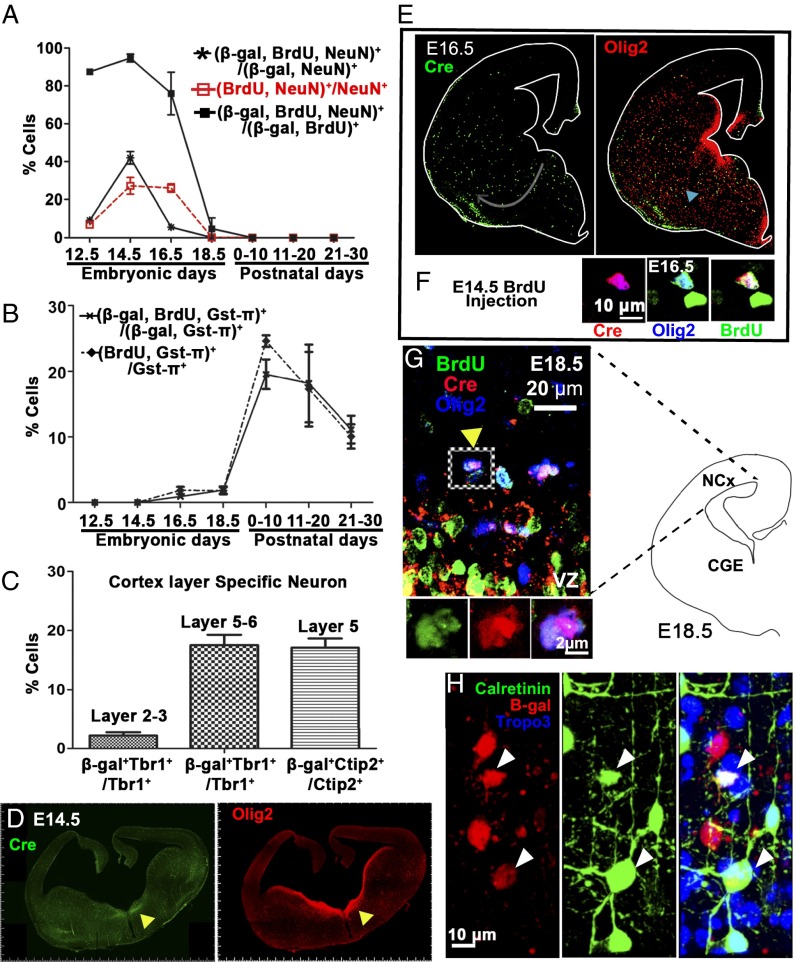
Our data thus far indicated that NG2 progenies found in the dorsal CTX were not exclusively GST-π+ and, thus, likely were not OLs but were some other terminally differentiated neural cell type. From the staining analysis, we found that a significant fraction of NG2 progenies was cortical neurons (Fig. S2 A–C). On the basis of our in situ hybridization and immunostaining analyses, we could not find expression of either Cre or CSPG4/NG2 in SVZ or VZ during early embryonic cortical development (E12.5–E16.5). This indicated that the NG2 progenitor-derived neurons do not come from dorsal cortical VZ/SVZ embryonic progenitor zones. However, administration of BrdU to postnatal Ng2-Cre/Rosa26LacZ mice did not label any postnatally generated neurons derived from NG2 progenitors (Fig. 3A), suggesting that these neurons must have come from an embryonic progenitor zone that is distinct from the cortical VZ/SVZ. Using layer-specific markers, Tbr1 and Ctip2, we demarcated each cortical layer and demonstrated that 17.1–19.0% of the neurons in layers V and VI were NG2-derived neurons, but only 2.15% ± 0.590% of neurons in layers 2 and 3 were derived from NG2 progenitors (Fig. 3C). During cerebral cortical development, the deep layer pyramidal neurons are typically generated earlier than pyramidal neurons in layers 2 and 3, and cortical NPCs, which give rise to layer-specific pyramidal cortical projection neurons, are located in the VZ. To accurately assess the timing of this particular neurogenesis from NG2+ progenitors, we pulse-labeled dividing cells by BrdU injection at E12.5, E14.5, E16.5, or E18.5 and collected tissues at P30. By costaining β-gal, neuronal lineage marker, and BrdU, we were able to determine when NG2-derived neurons were generated from the embryonic progenitor pool. As expected, BrdU staining indicated that in the dorsal CTX, the majority of pyramidal projection neurons generated at E12.5 specifically populate deep cortical layers, whereas neurons generated at E14.5 and E16.5 constituted most of the outer layers (19). β-gal and BrdU costaining showed that the peak in neurogenesis from NG2+ progenitors at E14.5 coincided with the highest proportion of labeled β-gal+ neurons, suggesting that these neurons were committing to a neuronal lineage and becoming postmitotic at E14 (Fig. 3A). This was very different from what we observed with NG2-derived OLs. We noticed that BrdU injection before E18.5 did not label any NG2-derived OLs either in CC or dorsal CTX (Fig. 3B). Again, this suggested that NG2-derived neurons compared with OLs do not share a common differentiation profile. Our results collectively demonstrate that most NG2+ progenitor cells that become postmitotic before E16.5 preferentially take on a neuronal fate, whereas NG2+ progenitors after E18.5 produce OLs.
Fig. 3.
Embryonically generated NG2 progenitor cells originated at MGE give rise to interneurons in mature dorsal CTX. (A and B) Pulse-chase BrdU labeling of embryonic NG2 progenitor cells at E12.5, E14.5, E16.5, and E18.5. Later, brain sections of Ng2-Cre/Rosa26LacZ were collected at P30 and stained with β-gal, neuronal lineage marker, or GST-π. “Birth date” and percentage of NG2 progenies were estimated and quantified by tracing BrdU-labeled neurons/OLs. The majority of NG2-derived neurons were generated at E14.5 (A), whereas NG2-derived OLs were generated postnatally (B). (C) Staining of β-gal+;neuronal lineage marker+ cells in dorsal CTX shows that NG2 progenies constitute 17.1–19.0% of neurons in layers 5 and 6, yet only 2.2% in layers 2–4 (Fig. S2C). (D) Costaining of E14.5 sections with Cre and Olig2 showing that the NG2+ progenitor cells were located at MGE, but not in cortical plate, during early embryonic development. These NG2/Cre+ progenitor cells were found at the nonprogenitor zone of MGE. (E) Few Olig2/Cre+ progenitor cells located near dorsal cortical VZ were BrdU+ after a 3-h exposure at E18.5. (F and G) BrdU labeling indicated that E14.5-generated NG2 progenitor cells migrated out from the MGE by E16.5. BrdU was administered at E14.5, and sections were collected at E16.5. (G) The enlarged confocal image of cells is indicated by the blue arrowhead in F. The migration path of NG2 progenitors is show2n in F. (H) β-gal+ cells were found to be Calretinin+ in mature dorsal CTX. For BrdU birthdating experimental time point, see Fig. S3.
NG2+ Progenitors Generated in the Ventral Cortical Regions During Early Embryonic Dates Differentiate into Cortical Interneurons.
We had found that NG2-derived neurons were not typical pyramidal neurons generated from embryonic VZ/SVZ regions, so we next searched for the origins of these neurons. As shown, the majority of dorsal cortical NG2-derived neurons became postmitotic at E14.5. Therefore, we looked for the location/source of NG2+ progenitors in E14.5 brain tissues and found them in the MGE in the basal ganglia by immunolabeling (Fig. 3D). Furthermore, these NG2+ cells costained with Olig2 (Fig. 3D and Fig. S2D), which suggested that neurogenic NG2+ progenitor cells were likely part of the Olig2+ neural lineage cells derived from the MGE. As mentioned, we did not find any NG2+ progenitors in the dorsal cortical VZ at E14.5 or earlier (Fig. 3D). Instead, proliferative NG2+ progenitors started to appear in the cortical VZ and SVZ only after E18.5, when gliogenesis was in motion (Fig. 3G).
Unlike Olig2+;NG2− VZ progenitors, NG2+ progenitor cells that were also Olig2+ at early embryonic stage (e.g., E14.5) were juxtaposed to MGE ventricular zones, ventrally and medially. From BrdU labeling at E14.5 and tracing BrdU+ cells at E14.5 and E16.5, we found that the majority of the Olig2+;NG2+ progenitor cells migrated out of the VZ of MGE and remained Olig2+ (Fig. 3 D–F), even after 2 d of migration (at E16.5). Unlike cortical projection neurons, which derive from the dorsal telencephalon (20), cortical interneurons derive from the subpallial telencephalon in the basal ganglia (21). Fate-mapping and interneuron migration studies have shown that embryonic (E12.5–E16.5) progenitor cells located within MGE and caudal ganglionic eminence are the primary source of cortical interneurons (22). These interneurons originate in the subpallial telencephalon and later migrate tangentially to reach their final destination in the cortical plate.
On the basis of the birth timing and migration pattern of NG2 progenitors in MGE, (Fig. 3 A and B), we speculated that these progenitor cells give rise to cortical interneurons, which were the neuronal types we had detected (Fig. 3A). By using Calretinin and β-gal immunostaining, we confirmed that a subset of interneuron lineage in the dorsal CTX was derived from the NG2 lineage that originated from MGE (Fig. 3H). To further characterize the NG2-derived cortical interneuronal populations, we also used antibodies against Parvalbumin (PV) and Somatostatin (two other markers for interneuronal subpopulations). We found that embryonic MGE-derived NG2 progenies were colabeled with PV, but not with Somatostatin (Fig. S1F). Previous studies suggested that ventral MGE progenitor zones preferentially gave rise to cortical PV+ interneurons, whereas dorsal MGE progenitor zones preferentially generated Somatostatin+ interneurons (23). Our results suggest that the majority of NG2+ progenitors are originated from the ventral MGE progenitor zone.
Our birth dating analysis suggested that the NG2+/Olig2+ populations found in MGE were progenitor cells that later gave rise to interneuronal populations found in the mature dorsal CTX. More than 90% of β-gal+ cells that were BrdU-labeled at E14.5 become neurons in the dorsal CTX, with the rest being nonterminally differentiated NG2+ cells. Analysis of BrdU labeling at E14.5 or E16.5 (3 h postlabeling) showed that in both cases, BrdU incorporation was restricted to progenitor regions of the ganglionic eminence. As mentioned earlier, one of the questions regarding oligodendrogliogenesis is the origin of OPCs. Our data show that NG2 postnatal progenitors in SVZ only give rise to OLs in both white and gray matters in cortical regions. Therefore, we asked whether NG2 embryonic progenitors found in MGE likewise migrate to dorsal cortical SVZ. To test this hypothesis, we labeled NG2 cells with BrdU at E14.5 and waited for 2 d to observe their migration. We found that NG2+ progenitors generated from MGE at this embryonic date follow a classical interneuronal tangential migration path (Fig. 3 E and F). Because we did not find any E14.5 BrdU-labeled NG2 cells in the dorsal cortical SVZ 2 d after labeling, this suggested that the migrating NG2 cells did not translocate to the dorsal cortical OPC zone (Fig. 3 E and F). Therefore, we concluded that embryonic NG2+ progenitors, which are located in MGE, and postnatal NG2+ progenitors in dorsal cortical SVZ do not originate from the same population of cells. Immunostaining for Calretinin, PV, and β-gal confirmed that embryonic NG2+ progenitors migrate to dorsal CTX and contribute to some interneuron generation, but not to OLs. More important, our results demonstrate that NG2+ progenitors come from different developmental origins. Depending on the progenitor domain from where they originate, these cortical interneuronal progenitors were restricted in their lineage choices, as suggested by the expression of different transcription factors (24), and their differentiation potentials were limited by these spatiotemporal specificities, as we observed in our Ng2-Cre/Rosa26LacZ double-transgenic mouse model.
Postnatal SVZ GFAP+ NPCs Are Precursors of NG2+ OPCs.
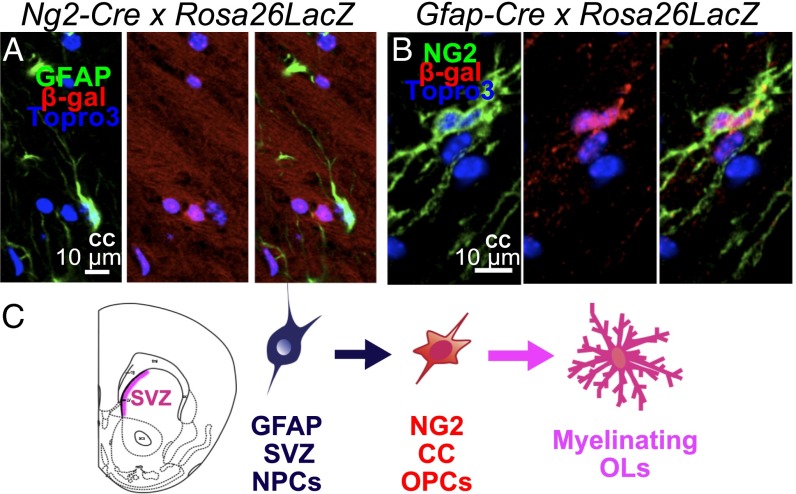
To examine whether NG2+ progenies labeled with GFAP marker are adult NPCs without SRY-box 2 expression or whether they are GFAP+ astrocytes, we first need to understand the connections between NG2+ cells and GFAP+ cells found in SVZ. All NPCs have two major characteristics: self-renewal and generating trineural lineages. The adult GFAP+ NPCs found in SVZ were documented to fulfill both of these criteria (25). Following this stream of thought, GFAP+ NPCs can gain the expression of NG2 and subsequently differentiate into myelinating OLs in the CC. A previous report using another line of NG2-Cre transgenic mice for lineage tracing proposed that NG2 cells give rise to cortical astrocytes in the gray matter (26). This observation suggested an alternative fate path: If NG2+ progenitors were the cells that later differentiated into GFAP+ astrocytes, then the NG2+ progenitors would gain the expression of GFAP during astroglial differentiation. In the first scenario, staining of GFAP and β-gal in Ng2-Cre/Rosa26LacZ double-transgenic mouse could not capture the GFAP+ NPC population. However, immunolabeling of NG2 and β-gal in mGFAP-Cre/Rosa26LacZ double-transgenic mice would reveal such a population in the SVZ. As we revisited the relationship between NG2+ and GFAP+ cells in CC of our Ng2-Cre/Rosa26LacZ double-transgenic mouse, with GFAP and β-gal immunostaining, we did not detect any GFAP+ astrocytes coexpressing β-gal within CC or deep cortical layers of P30 mice (Fig. 4A), similar to the tracing result using PdgfαR-CreER/Rosa26YFP (17). Furthermore, a most recent report with a StarTrack-labeled pallial NG2 population suggested that the SVZ-originated NG2 progenitors, although giving rise to the largest clonal oligodendrocyte clusters in the CTX and olfactory bulb, lack astroglial potential in vivo (27). This indicated that NG2+ progenitors in CC do not become GFAP+ astrocytes. Conversely, to address whether NG2+ progenitors in CC are derived from GFAP+ NPCs in the subependymal zone of the forebrain, we used mGFAP-Cre/Rosa26LacZ double-transgenic mice (28). At P30, β-gal staining to trace the GFAP+ progenitor lineage in the CC showed costaining with NG2 cells (Fig. 4B). This finding was in agreement with the previous reports indicating that GFAP+ NPCs found in postnatal SVZ give rise to OPCs, which differentiate into OLs in both dorsal CTX and CC (Fig. 4C).
Fig. 4.
The postnatal NG2 progenitor cells found in CC came from SVZ GFAP+ progenitor source. (A) Immunostaining of β-gal and GFAP in Ng2-Cre/Rosa26LacZ double-transgenic mouse brain sections showed that GFAP+ astrocytes in CC are not derived from NG2 progenitors. (B) Immunostaining of β-gal and NG2 in Gfap-Cre/Rosa26LacZ double-transgenic mouse brain sections showed that NG2+ cells within CC were derived from GFAP+ postnatal NPCs. (C) The schematic order of lineage differentiation from GFAP+ NPCs to NG2+ OPCs to myelinating OLs.
Discussion
To identify cellular functions and cell types, developmental biologists have historically relied on techniques that use cell markers, such as NG2, to determine cell fate potentials. However, because of the power of current sequencing technology and bioinformatics, new evidence for the true complexity of cell identity, especially in the CNS, has been established. The differentiation of specific neural cell types is highly influenced by spatial and temporal cues during early development. Spatial regulation is imposed starting within the progenitor zone, where a particular progenitor cell originates.
Different Pools of NG2+ Progenitor Cells in Embryonic and Postnatal CNS.
On the basis of work from chick-quail grafting, it was first suggested that OLs in the forebrain originated from OPCs in the ventral telencephalon (or anterior entopeduncular area) (29). Later, lineage-mapping studies using different promoter-driven Cre (Nkx2.1, Gsh2, and Emx1) suggested three OPC origins. The earliest MGE PDGFαR+ progenitor cells were identified as the first OPC pool during the E12.5 developmental stage. The second PDGFαR+ progenitor pool originated from lateral ganglionic eminence and caudal ganglionic eminence. The last PDGFαR+ progenitor pool was located at the dorsal cortical VZ/SVZ during the neonatal stage (15). From our BrdU incorporation and Ng2-Cre/Rosa26LacZ tracing analyses, we have revealed the terminal fate differences between the three separate NG2+ progenitor pools, depending on their temporal origins. Our genetic NG2 lineage tracing approach, together with detailed pre- and postnatal BrdU labeling and BrdU pulse-chasing, demonstrated that there are different pools of NG2+ progenitors based on their terminal cell fates: in the ventral MGE during midgestation stages and in dorsal cortical VZ/SVZ at perinatal and postnatal stages (E18.5 and later). Unlike the previous assertion that all NG2+ cells have only one differentiation capacity (i.e., oligodendroglial lineage), our study applied multiple strategies to determine that neonatal/postnatal NG2+ progenitors that originated in the dorsal SVZ are the only NG2+ progenitor pool that has oligodendroglial lineage potential.
Generation of Cortical Interneurons from NG2+ Progenitors.
Earlier ex vivo studies demonstrated that perinatal SVZ NG2+ cells isolated from Cnp-GFP transgenic mouse were capable of generating GABAergic interneurons, which was the first report that questioned the traditional view that NG2 progenitors exclusively give rise to OPCs (13, 14). Our BrdU pulse-labeling analysis indicated that MGE-originated early embryonic NG2+;PDGFαR+ progenitor cells, while expressing Olig2, become postmitotic when migrating out of the MGE. Furthermore, our results indicated that this early MGE NG2+ progenitor pool is not the progenitor source for OLs found in dorsal CTX and CC. Instead, this population gave rise to some GABAergic interneurons in dorsal cortical regions through a tangential migration path. This result suggests an alternative to the theory of the so-called “ventral origin” of oligodendrogliogenesis. One may argue that progenitors derived from embryonic MGE were the source of NG2+ progenitors in the neonatal SVZ. However, our BrdU and tracing analyses indicated that NG2+ progenitors that arise from the embryonic MGE tangentially migrate out of the origin zone instead of translocating to the SVZ region (Fig. 3 D–G). Therefore, the NG2+ progenitors found in neonatal SVZ must be derived from an origin different from the population that differentiates into interneurons. Considering that NG2/PDGFRα/Olig2 progenitor cells are detected in different locations (i.e., embryonic MGE and postnatal SVZ) at different developmental periods, any effort to deduce their terminal cell fate must use detailed birth-dating and lineage development analyses in a spatiotemporal manner. Without following the migration pattern of these embryonically generated NG2+ progenitor populations through early development and characterizing the layer specificity bias in the cortical zone, the interneuronal fate of NG2+ progenitors could be easily missed. This may explain why, in other studies, it was believed that the postnatal NG2+ progenitors derived from SVZ diluted the embryonically generated populations (15).
Mechanisms of Cell Fate Choice by Different NG2+ Progenitors.
BrdU pulse-chase, along with lineage-mapping studies of NG2+ progenitor populations, showed a diverse differentiation capacity of NG2+ cells, highlighting the complexity and heterogeneity of the NG2+ progenitor populations. During early developmental stages, the gradient established by sonic hedgehog, fibroblast growth factors, bone morphogenetic proteins, and WNT (proteins that form a family of highly conserved secreted signaling molecules that regulate cell-to-cell interactions) signaling establishes progenitor zones in the CNS. Within each progenitor zone, a combination of expressed neural transcription factors leads a cell to a specific lineage preference. Unlike neurogenesis, which mainly takes place during early embryonic dates, oligodendrogliogenesis first occurs at the neonatal stage, as well as from late postnatal OPCs that can remyelinate in response to CNS injury. At these later development times, microenvironmental cues can change drastically from one cortical region to another. Therefore, to direct the proper oligodendroglial differentiation, the OPCs must rely on their own intrinsic regulatory elements instead of environmental signals. Our preliminary analysis on transcription factor expressions also suggested such diversity between various NG2+ populations on the basis of spatial and temporal origins. It is likely that the injury site secretes signals to attract OPCs, but the differentiation programs would still depend on the intrinsic regulation.
Our interest in the origins and functions of NG2+;PDGFαR+ OPCs is based not only on their capacity to differentiate into OLs but also on their response to injury and their role in diseases, such as multiple sclerosis in the postnatal brain (30). From our data, we observed spatial and temporal diversity between NG2+ progenitors beyond those seen between progenitor origins found in embryonic MGE and postnatal SVZ regions. In fact, the classically defined OPC lineage is suitable to describe only the NG2+ progenitor cells generated close in time to the neonatal stage and within the SVZ. The important role of these spatially and temporally specific regulatory processes is further supported by the fact that different postnatal NG2+;PDGFαR+ progenitor pools have diverse lineage outcomes (Fig. 5) and electrophysiological properties (15, 31–33).
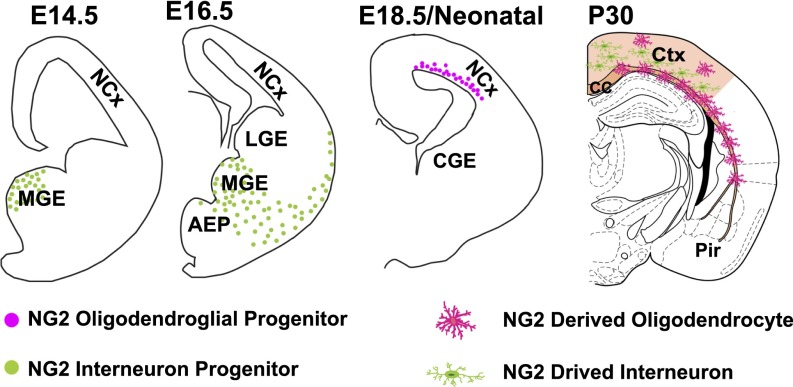
Fig. 5.
The origins of two subgroups of NG2 progenitors during development in relation to their final terminal cell fate and location. At E14.5, the first-wave (newly generated) NG2+ progenitor cells are located at the MGE. At E16.5, the E14.5-generated NG2+ interneuronal progenitors tangentially migrate out of the MGE progenitor zone toward the cortical marginal layer. At E18.5, the late embryonic/neonatal GFAP+ NPC-derived NG2+ OPCs appear at the lateral ganglionic eminence (dorsal VZ and SVZ). At P30, the mature NG2 derived interneurons reside within dorsal cortical deep layers. The mature NG2 derived OLs found in both gray and white matters.
In this study, we have shown that although distinct progenitor populations share the same recognition marker, NG2, the differences in lineage programming between populations isolated from different spatial and temporal origins is predictive of diverse lineage potentials. NG2+ progenitors derived from the MGE region are programmed to differentiate into cortical interneurons, yet the NG2+ progenitors that arise from postnatal SVZ only contribute to OL lineages. Collectively, our results indicate that each individual NG2+ progenitor cell takes on a different neural lineage fate and acts as a lineage-restricted progenitor in a spatially and temporally specific manner (Fig. 5).
Materials and Methods
The animals were housed in the University of California, Los Angeles animal care facility based on the guidelines approved by the Animal Research Committee at the University of California, Los Angeles. Immunohistochemistry sections were imaged using a confocal microscope (Zeiss LSM 510 Meta). Between four and six sections were evaluated per mouse for each staining, and three mice were analyzed for each age indicated. Significance was measured using a two-way ANOVA test. Further details are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the University of California, Los Angeles, Intellectual and Developmental Disabilities Research Center, Epigenetics Core and Stem Cell Core for technical support. We acknowledge Major State Basic Research Development Program of China Grant 2010CB945202 (to Y.E.S.). This work was supported by grants from the National Institutes of Health [2 P30-HD004612 and P30-HD06576 (to J.d.V.) and P01 GM081621-01A1 and 1R01MH082068-01A2 (to Y.E.S.)] and California Institute of Regenerative Medicine (RB3-02129 to Y.E.S.). Y.E.S. is also supported by National Institutes of Health Grant P50DA005010.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400422111/-/DCSupplemental.
References
- 1.Calegari F, Haubensak W, Haffner C, Huttner WB. Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain development. J Neurosci. 2005;25(28):6533–6538. doi: 10.1523/JNEUROSCI.0778-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409(6821):714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 3.Ross SE, Greenberg ME, Stiles CD. Basic helix-loop-helix factors in cortical development. Neuron. 2003;39(1):13–25. doi: 10.1016/s0896-6273(03)00365-9. [DOI] [PubMed] [Google Scholar]
- 4.Sauvageot CM, Stiles CD. Molecular mechanisms controlling cortical gliogenesis. Curr Opin Neurobiol. 2002;12(3):244–249. doi: 10.1016/s0959-4388(02)00322-7. [DOI] [PubMed] [Google Scholar]
- 5.Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;7(1):11–18. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spassky N, et al. Multiple restricted origin of oligodendrocytes. J Neurosci. 1998;18(20):8331–8343. doi: 10.1523/JNEUROSCI.18-20-08331.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spassky N, et al. Single or multiple oligodendroglial lineages: A controversy. Glia. 2000;29(2):143–148. [PubMed] [Google Scholar]
- 8.Rowitch DH. Glial specification in the vertebrate neural tube. Nat Rev Neurosci. 2004;5(5):409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- 9.Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5(12):1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- 10.Matta JA, et al. Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat Neurosci. 2013;16(8):1032–1041. doi: 10.1038/nn.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rallu M, Corbin JG, Fishell G. Parsing the prosencephalon. Nat Rev Neurosci. 2002;3(12):943–951. doi: 10.1038/nrn989. [DOI] [PubMed] [Google Scholar]
- 12.Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561(Pt 1):109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165(4):575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belachew S, et al. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161(1):169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kessaris N, et al. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9(2):173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson WD, Young KM, Tripathi RB, McKenzie I. NG2-glia as multipotent neural stem cells: Fact or fantasy? Neuron. 2011;70(4):661–673. doi: 10.1016/j.neuron.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivers LE, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11(12):1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robins SC, et al. Extensive regenerative plasticity among adult NG2-glia populations is exclusively based on self-renewal. Glia. 2013;61(10):1735–1747. doi: 10.1002/glia.22554. [DOI] [PubMed] [Google Scholar]
- 19.Rakic P. Specification of cerebral cortical areas. Science. 1988;241(4862):170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 20.Chan C-H, et al. Emx1 is a marker for pyramidal neurons of the cerebral cortex. Cereb Cortex. 2001;11(12):1191–1198. doi: 10.1093/cercor/11.12.1191. [DOI] [PubMed] [Google Scholar]
- 21.Marín O, Rubenstein JLR. A long, remarkable journey: Tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2(11):780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 22.Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128(19):3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- 23.Flames N, et al. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27(36):9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma T, et al. Subcortical origins of human and monkey neocortical interneurons. Nat Neurosci. 2013;16(11):1588–1597. doi: 10.1038/nn.3536. [DOI] [PubMed] [Google Scholar]
- 25.Suh H, et al. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1(5):515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135(1):145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 27.García-Marqués J, Núñez-Llaves R, López-Mascaraque L. NG2-glia from pallial progenitors produce the largest clonal clusters of the brain: Time frame of clonal generation in cortex and olfactory bulb. J Neurosci. 2014;34(6):2305–2313. doi: 10.1523/JNEUROSCI.3060-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia ADR, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7(11):1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 29.Olivier C, et al. Monofocal origin of telencephalic oligodendrocytes in the anterior entopeduncular area of the chick embryo. Development. 2001;128(10):1757–1769. doi: 10.1242/dev.128.10.1757. [DOI] [PubMed] [Google Scholar]
- 30.Wilson HC, Scolding NJ, Raine CS. Co-expression of PDGF α receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J Neuroimmunol. 2006;176(1-2):162–173. doi: 10.1016/j.jneuroim.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Tamura Y, et al. Multi-directional differentiation of doublecortin- and NG2-immunopositive progenitor cells in the adult rat neocortex in vivo. Eur J Neurosci. 2007;25(12):3489–3498. doi: 10.1111/j.1460-9568.2007.05617.x. [DOI] [PubMed] [Google Scholar]
- 32.Káradóttir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11(4):450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarke LE, et al. Properties and fate of oligodendrocyte progenitor cells in the corpus callosum, motor cortex, and piriform cortex of the mouse. J Neurosci. 2012;32(24):8173–8185. doi: 10.1523/JNEUROSCI.0928-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.