Figure 12.1.
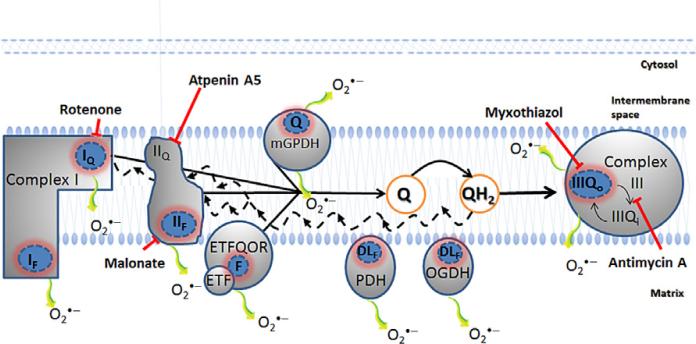
Eight sites of mitochondrial superoxide/H2O2 production are identified. Complex I oxidizes NADH from the tricarboxylic acid cycle and produces superoxide at the flavin site (IF) and ubiquinone-binding site (IQ), both sites release superoxide to the matrix side. Complex II, a tricarboxylic acid cycle enzyme bound to the mitochondrial inner membrane, oxidizes succinate to fumarate and generates superoxide/H2O2 both in the reverse and forward reactions through the flavin site (IIF). Glycerol 3-phosphate dehydrogenase (mGPDH) is part of an important shuttle for oxidation of cytosolic NADH and releases superoxide to both matrix and cytosol. The electron-transferring flavoprotein (ETF) and electron-transferring flavoprotein ubiquinone oxidoreductase (ETFQOR) represent the final steps for β-oxidation; it is not known which of the two sites is responsible for superoxide/H2O2 production during β-oxidation. The IIIQo site of complex III is also a source of superoxide to both cytosol and matrix. In addition to the electron transport chain sites, pyruvate dehydrogenase (PDH) and oxoglutarate dehydrogenase (OGDH) both contain the dihydrolipoamide (DL) dehydrogenase subunit that can generate super-oxide/H2O2, possibly through the DL or flavin moieties. The black solid line shows complex I, complex II, mGPDH, and ETFQOR feeding electrons into the ubiquinone (Q)-pool. With a very reduced Q-pool (QH2), the electrons can run in reverse through complex I and complex II, promoting ROS generation at sites IQ, IF, and IIF (black dashed lines). The blunted arrows indicate the sites where the inhibitors rotenone, malonate, atpenin A5, myxothiazol, and antimycin A bind. The use of such inhibitors is necessary to pharmacologically isolate the sites of ROS production from each other.