Abstract
Background and Objectives
This study was performed to investigate recent trends and factors associated with immediate breast reconstruction (IBR) using a large population-based registry. We hypothesized that rates of IBR have increased since passage of the Women’s Health and Cancer Rights Act of 1998.
Methods
The SEER (Surveillance, Epidemiology and End Results) database was used to evaluate Stage I–III breast cancer (BC) patients who underwent total mastectomy from 1998–2008. Univariate and multivariate analyses were performed to study predictors of IBR.
Results
Of 112,348 patients with BC treated by mastectomy, 18,001 (16%) had IBR. Rates of IBR increased significantly from 1998–2008 (p<0.0001). Use of IBR significantly decreased as patient age increased (p<0.0001), as stage increased (p<0.0001), and as the number of positive lymph nodes increased (p<0.0001). Estrogen receptor +/progesterone receptor + (ER+/PR+) patients had significantly higher IBR rates than ER−/PR− patients (p<0.0001). IBR was used in 3615 of 25,823 (14.0%) of patients having post-mastectomy radiation (XRT) and in 14,188 of 86,513 (16.4%) of those not having XRT (p<0.0001).
Conclusions
The utilization of IBR has increased significantly over the last decade. IBR was found to be significantly associated with age, race, geographical region, stage, ER, grade, LN status, and XRT (p<0.0001).
Keywords: SEER, breast cancer, mastectomy, immediate breast reconstruction
INTRODUCTION
Approximately 230,500 women were diagnosed with invasive breast cancer during 2011 in the United States[1]. Historically, approximately one-third of breast cancer patients have been treated with mastectomy and the remaining two-thirds had breast-conserving surgery,[2,3] resulting in a possible 76,833 potential candidates for reconstruction in 2011. About 17% of women undergoing mastectomy from 1998 to 2002 had either immediate or delayed breast reconstruction[4]. Immediate breast reconstruction (IBR) has increasingly gained clinical acceptance as an integral component of multidisciplinary care, and is generally considered a standard of care approach for appropriately selected mastectomy patients. IBR has the advantage of retaining the skin envelope, which results in improved aesthetic outcomes, fewer operations to achieve the reconstructive goals, and potential psychological benefits to the patient[5]. The risk of masking recurrence with breast reconstruction is minimal[6–9].
However, IBR has been shown to have higher complication rates, approaching 40% compared to 17% for delayed breast reconstruction[10,11]. Our institution has previously reported that immediate breast reconstruction is associated with a statistically significant increased risk of surgical site infection in patients undergoing mastectomy (3.5% vs. 2.5%)[12]. Complications may increase the time interval required for wound healing and possibly delay the delivery of adjuvant chemotherapy or radiotherapy (XRT)[13]. Although Alderman et al. demonstrated that IBR was associated with a modest but statistically significant delay in initiating chemotherapy, Rey et al. and Peled et al. reported that IBR was not associated with increased complications and did not cause a delay in chemotherapy[14,15]. A primary factor involved with the decision to utilize IBR is post-mastectomy radiation, which may compromise the results of both autologous and implant-based reconstructions and limit use of IBR in patients for whom post-mastectomy radiation is anticipated[16–18]. Furthermore, Alderman et al. found that only one-third of breast cancer patients reported that a general surgeon discussed the option of breast reconstruction with them at the time of surgical decision-making, suggesting that selection bias may be an important barrier to IBR[19]. The rates of post-mastectomy IBR are also affected by other variables, such as patient comorbidities, obesity, smoking, and personal preferences[20,21].
The Surveillance, Epidemiology and End Results (SEER) database of the National Cancer Institute contains data from about 28% of the breast cancer patients diagnosed annually in the United States, and it has previously been used to study trends in breast reconstruction[20,22,23],[24]. Previous evaluations of the SEER database from 1998 to 2002, did not demonstrate increased rates of breast reconstruction following passage of the Women’s Health and Cancer Rights Act of 1998[24,25]. These studies did, however, detect differences in rates of IBR associated with age, race, geographical location, and XRT. The purpose of this study was to examine trends in rates of IBR using this more contemporary SEER cohort, from 1998 to 2008[24]. It should be noted that, although the SEER 13 database did not report receipt of chemotherapy, endocrine therapy, or trastuzumab, prior analyses have demonstrated that ER status did correlate with the probability of using adjuvant systemic therapy26–28.
We hypothesized that rates of IBR have increased since passage of the Women’s Health and Cancer Rights Act of 1998. A secondary aim of our study was to identify patient, tumor, treatment and geographical factors correlated with use of IBR in this large population-based study of patients treated by mastectomy for newly diagnosed Stage I–III breast cancer.
PATIENTS AND METHODS
De-identified patient information for the years 1998–2008 was collected from the prospectively maintained SEER registry and included in our study. The SEER 17 registry was used for our analysis and this includes data from the SEER 13 registry (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah, Los Angeles, San Jose-Monterey, Rural Georgia and the Alaska Native Tumor Registry), plus Greater California, Kentucky, Louisiana and New Jersey. The patients in the Alaska Native Tumor Registry were excluded since a significant proportion of their patients receive IBR out of state, if needed. Patients were classified according to five racial groups: White, Black, American Indian/Alaska Native, Asian/Pacific Islander, and Hispanic origin.
Three types of variables were included in the analysis: 1) patient demographics- age, race, and geographical region; 2) tumor characteristics-ER/PR status, axillary lymph node status, histologic type, tumor grade, and tumor stage; and 3) treatment variables-use of XRT and IBR. Reconstruction within the first 4 months after mastectomy was defined as IBR, according to the SEER database field code definitions. Data regarding the distinction between immediate and early-delayed reconstruction were not available in the SEER database, nor were there data on reconstruction delayed more than 4 months post-mastectomy. Reconstructive procedures exclusively associated with the contralateral breast were excluded from our study.
Inclusion and exclusion criteria
Female patients with a new diagnosis of breast cancer who underwent mastectomy for Stage I–III breast cancer between the years 1998–2008 were included in the study. Patients who had subcutaneous mastectomy (code 30), simple mastectomy (40–49, 75), modified radical mastectomy (50–59, 63), radical mastectomy (60–62, 64–69, 73–74) and mastectomy (80) were included in the study. Patients who had partial mastectomy or extended radical mastectomy were excluded from the study. Patients who had any prior history of any type of cancer were also excluded (n=26,511). Patients with invasive ductal, lobular, or combined ductal and lobular carcinoma were included, but those with purely in-situ ductal or lobular carcinoma were excluded. Those with Stage IV breast cancer or a diagnosis of inflammatory breast cancer were excluded. Patients who had no data regarding ER/PR status or XRT were excluded. Follow up data through 2010 was available for analysis.
Statistical Analysis
The Chi-squared test was used to examine the association between reconstruction rate and patient/tumor characteristics. The relative risk was estimated for each variable. The Cochran-Armitage trend test was performed to investigate the underlying trend for reconstruction rate. For the multivariate analysis, the logistic regression model was employed to assess predictive factors for breast reconstruction. For each variable, adjusted relative risk (RR) with 95% confidence intervals (CIs) were computed. All statistical analyses were performed with SAS 9.2 (SAS Institute Inc., Cary, NC). Tests were deemed to be significant at a p-value of 0.05.
RESULTS
Patient and Disease Characteristics
From January 1, 1998–December 31, 2008, there were 371,309 patients diagnosed with breast cancer who had data recorded in SEER 13. Of these, 112,348 patients (30.2%) had mastectomy and met all inclusion/exclusion criteria. There were 18,001 (16.0%) patients had who IBR after mastectomy. Table I summarizes the patient demographics and tumor characteristics of the study population.
Table I.
Patient demographics and tumor characteristics
| Characteristics | Number | % |
|---|---|---|
| Age | ||
| 20–29 years | 797 | 0.7 |
| 30–39 years | 8092 | 7.2 |
| 40–49 years | 24,105 | 21.5 |
| 50–59 years | 26,842 | 23.9 |
| 60–69 years | 22,294 | 19.8 |
| 70–79 years | 19,038 | 17.0 |
| 80+ years | 11,180 | 10.0 |
| Race/ethnicity | ||
| White | 80,325 | 71.8 |
| Black | 10,120 | 9.1 |
| American Indian / Alaska Native | 401 | 0.4 |
| Asian or Pacific Islander | 9981 | 8.9 |
| Hispanic | ||
| Geographic Region | ||
| East | 34,411 | 30.6 |
| Northern Plains | 14,539 | 12.9 |
| Pacific Coast | 57,626 | 51.3 |
| Southwest | 5772 | 5.1 |
| Regional lymph nodes # positive | ||
| 0 | 57,483 | 51.2 |
| 1–4 | 34,585 | 30.8 |
| 5–10 | 10,790 | 9.6 |
| >10 | 9490 | 8.4 |
| Grade | ||
| 1 | 16,082 | 15.3 |
| 2 | 42,738 | 40.5 |
| 3 | 44,656 | 42.4 |
| 4 | 1943 | 1.8 |
| Stage | ||
| I | 37,796 | 34.8 |
| II | 54,140 | 49.9 |
| III | 16,643 | 15.3 |
| ER | ||
| Positive | 84,008 | 74.8 |
| Negative | 28,340 | 25.2 |
| PR | ||
| Positive | 69,251 | 63.1 |
| Negative | 40,557 | 36.9 |
| Radiation | ||
| Yes | 25,823 | 23.0 |
| No | 86,513 | 77.0 |
Annual IBR Rates
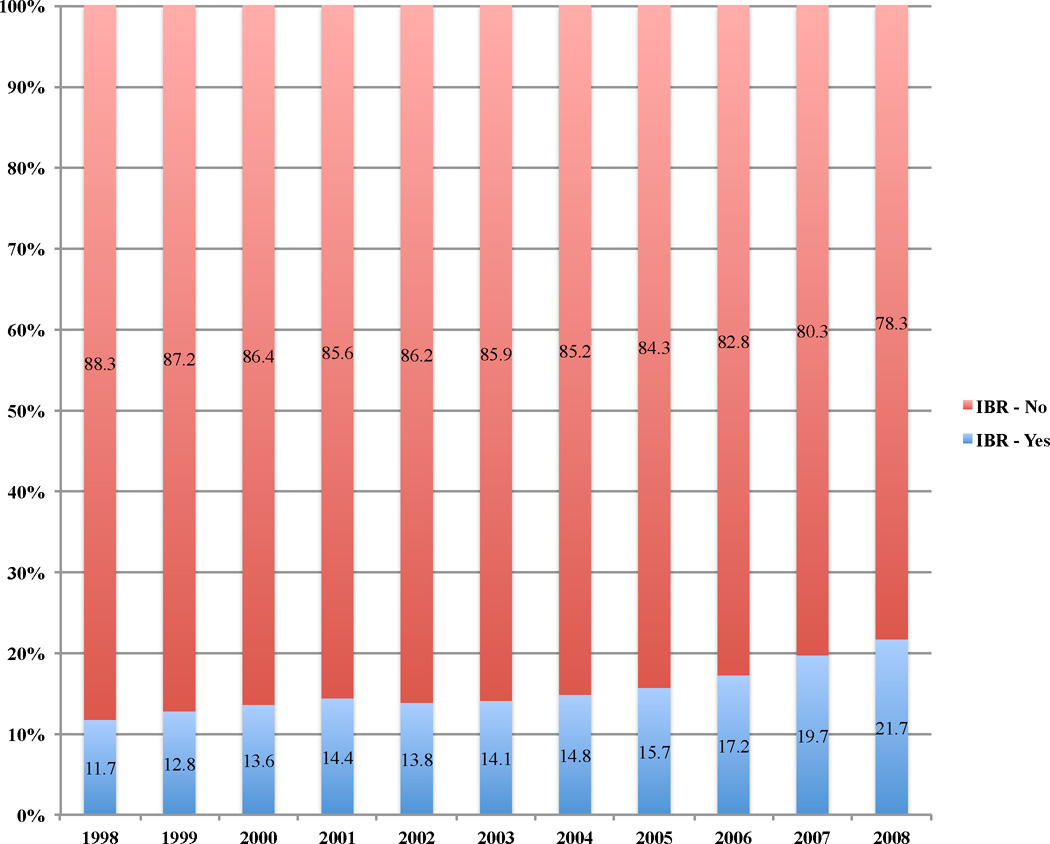
As demonstrated in Figure 1, IBR rates steadily increased over time, from 712 (11.7%) in 1998 to 2774 (21.7%) in 2008, a near doubling in rates during this timeframe (p<0.0001). A modest 2.1% increase in IBR rates was found from 1998 to 2002, with rates increasing from 11.7% to 13.8%. However, from 2002 to 2008 the rate increase was noted to be 7.9%, nearly 4 times the increase noted from 1998 to 2002, indicating that most of the increase in IBR rate was associated with the latter portion of the study.
Figure 1.
IBR rates increased from 1998–2008. A near doubling of rates of IBR occurred when comparing rates in 1998 to 2008.
Patient Variables
Univariate (Table II) and multivariate (Table III) analyses were performed correlating patient, tumor, and treatment variables with likelihood of IBR. IBR rates significantly decreased as patient age increased. Patients on the Pacific Coast and in the Southwest were significantly less likely to have IBR than those in the East. Non-white patients were significantly less likely to have IBR than White patients.
Table II.
Association of patient demographics and tumor characteristics with IBR (Univariate Analysis).
| Characteristics | Reconstruction Rate (%) |
P-value for the difference | P-value for the trend |
|---|---|---|---|
| Age | |||
| 20–29yrs | 32.6 | ||
| 30–39yrs | 30.5 | ||
| 40–49yrs | 27.6 | ||
| 50–59yrs | 20.1 | <0.0001 | <0.0001 |
| 60–69yrs | 10.1 | ||
| 70–79yrs | 3.3 | ||
| >80yrs | 0.8 | ||
| Geographical Region | |||
| East | 20.3 | ||
| Northern Plains | 18.7 | ||
| Pacific Coast | 12.4 | <0.0001 | NA |
| Southwest | 16.0 | ||
| Race/Ethnicity | |||
| White | 17.5 | ||
| Black | 13.4 | ||
| American Indian/Alaska Native | 10.0 | ||
| Asian or Pacific Islander | 9.5 | <0.0001 | NA |
| Hispanic | 11.8 | ||
| Stage | |||
| I | 19.0 | ||
| II | 15.3 | <0.0001 | <0.0001 |
| III | 11.4 | ||
| Grade | |||
| 1 | 16.8 | ||
| 2 | 16.2 | ||
| 3 | 15.4 | <0.0001 | <0.0001 |
| 4 | 15.0 | ||
| Radiation | |||
| No | 16.4 | ||
| Yes | 14.0 | <0.0001 | NA |
| Number of positive nodes | |||
| 0 | 17.3 | ||
| 1–4 | 16.2 | <0.0001 | <0.0001 |
| 5–10 | 11.8 | ||
| >10 | 10.1 | ||
| ER | |||
| Positive | 16.2 | NA | |
| Negative | 14.9 | <0.0001 | |
| PR | |||
| Positive | 16.8 | ||
| Negative | 14.5 | <0.0001 | NA |
| Tumor size | |||
| <2cm | 19.4 | ||
| 2–5cm | 13.9 | <0.0001 | <0.0001 |
| >5cm | 11.8 | ||
Table III.
Association of patient demographics and tumor characteristics with IBR (Multivariate Analysis).
| Characteristics | Adjusted RR | 95% CI | p-value |
|---|---|---|---|
| Age | |||
| 20–29 years | 1.00 | - | - |
| 30–39 years | 0.88 | (0.78, 0.98) | 0.02 |
| 40–49 years | 0.75 | (0.68, 0.83) | <0.0001 |
| 50–59 years | 0.53 | (0.48, 0.58) | <0.0001 |
| 60–69 years | 0.25 | (0.23, 0.28) | <0.0001 |
| 70–79 years | 0.08 | (0.07, 0.09) | <0.0001 |
| 80+ years | 0.02 | (0.01, 0.02) | <0.0001 |
| Race/ethnicity | |||
| White | 1.00 | - | - |
| Black | 0.65 | (0.61, 0.69) | <0.0001 |
| American Indian | 0.52 | (0.38, 0.70) | <0.0001 |
| Asian or Pacific Islander | 0.54 | (0.50, 0.58) | <0.0001 |
| Hispanic | 0.66 | (0.63, 0.69) | <0.0001 |
| Geographic Region | |||
| East | 1.00 | - | - |
| Northern Plains | 0.97 | (0.93, 1.003) | 0.07 |
| Pacific Coast | 0.67 | (0.65, 0.69) | <0.0001 |
| Southwest | 0.78 | (0.74, 0.84) | <0.0001 |
| Number of positive nodes | |||
| 0 | 1.00 | - | - |
| 1–4 | 0.96 | (0.92, 1.009) | 0.11 |
| 5–10 | 0.82 | (0.77, 0.88) | <0.0001 |
| >10 | 0.79 | (0.73, 0.85) | <0.0001 |
| Grade | |||
| 1 | 1.00 | - | - |
| 2 | 0.96 | (0.93, 1.009) | 0.06 |
| 3 | 0.90 | (0.87, 0.94) | 0.03 |
| 4 | 0.88 | (0.79, 0.98) | <0.0001 |
| Stage | |||
| I | 1.00 | - | - |
| II | 0.93 | (0.88, 0.98) | <0.0001 |
| III | 0.84 | (0.77, 0.91) | <0.0001 |
| Tumor size | |||
| <2cm | 1.00 | - | - |
| 2–5cm | 0.82 | (0.79, 0.85) | <0.0001 |
| >5cm | 0.75 | (0.71, 0.80) | <0.0001 |
| ER | |||
| Positive | 1.00 | - | |
| Negative | 0.89 | (0.86, 0.94) | <0.0001 |
| PR | |||
| Positive | 1.00 | - | - |
| Negative | 0.98 | (0.94, 1.02) | 0.33 |
| Radiation | |||
| No | 1.00 | - | - |
| Yes | 0.82 | (0.79, 0.85) | <0.0001 |
RR=relative risk
Tumor and Treatment Variables
Univariate (Table II) and multivariate (Table III) analyses were performed correlating tumor and treatment variables with likelihood of IBR. IBR rates significantly decreased as tumor size increased, grade increased, the number of positive nodes increased, and stage increased. While there was not a significant difference found in rates of IBR for patients with negative versus 1–4 positive nodes, the likelihood of IBR was decreased in patients with more positive nodes (5–10 positive nodes (RR-0.82, p<0.0001, (0.77–0.88)) and >10 positive nodes (RR-0.79, p<0.0001, (0.73–0.85))). Patients who were both ER+ and PR+ had a significantly higher rate of IBR (16.2%) when compared with patients who were ER− and PR− (14.5%) and with patients who were either ER+ or PR+ (13.9%) (p<0.0001 and p<0.0001, respectively). Patients with Stage I ER+/PR+ cancers were 1.9 times more likely to have IBR than those with Stage III ER−/PR− cancers (p<0.0001). Patients with Stage III ER+/PR+ cancers were 1.3 times more likely to have IBR than patients with Stage III ER−/PR− tumors (p<0.0001).
IBR and XRT
In this study population, IBR was performed in 3615 of 25,823 (14.0%) patients treated with post-mastectomy XRT and in 14,188 of 86,513 (16.4%) of those not having XRT (p<0.0001). Although the absolute difference in the rates of IBR was relatively small, a statistically significantly difference was found in rates of IBR between the radiated and non-radiated mastectomy patients.
DISCUSSION
The passage of the Women’s Health and Cancer Rights Act of 1998 laid the groundwork for increasing utilization of IBR in the United States, as this Federal legislation mandated that insurance companies offering mastectomy must also provide coverage for all stages of reconstruction29. The effects of this legislation was evaluated in the past by Alderman et al, who reported there was no significant increase in the annual rates of IBR between 1998–2002 based on the SEER registry[25]. In contrast, the current study has demonstrated an increase in immediate reconstruction rates from 1998 to 2008. The majority of the increase in this rate seems to have occurred after 2002, explaining why the earlier SEER analyses failed to note a difference in IBR rates. To our knowledge, this is the first SEER-based study to document this observation.
This nationwide trend has been apparent in other large population-based studies, including analysis of the Nationwide Inpatient Sample (37.8% IBR) by Albornoz et al.[26] as well as non-population based databases, such as the National Comprehensive Cancer Center Network consortium (42% IBR) by Christian et al.[27] However, the study by Albornoz et al., while capturing a large cross section of mastectomy treated patients, was heterogeneous in that it included patients who had prophylactic mastectomy without cancer diagnosis as well as patients with ductal carcinoma in situ (DCIS). Similarly, the study by Christian et al. included DCIS patients; as nearly all DCIS patients treated by mastectomy, in the absence of co-morbidities, could be offered immediate breast reconstruction without concern for the need for adjuvant radiotherapy, we purposely excluded this diagnosis for our analysis. The objective of the present study was to determine rates of IBR in an updated SEER cohort restricted to patients with newly diagnosed Stage I–III breast cancer, taking into consideration the effect of available relevant patient, tumor and treatment variables captured by the National Cancer Institute’s SEER registry. The study by Christian el al. reflects IBR rates at a subset of comprehensive cancer centers and thus the impressive rate of IBR reported may not reflect national practice patterns for rates of IBR in community-based practice.
The increased rates of IBR may provide insight into changing nationwide trends in multidisciplinary oncological care from 1998–2008, such as increasing use of neoadjuvant treatment, skin-sparing mastectomy, and multidisciplinary decision-making prior to therapy (tumor boards). The advent of skin-sparing mastectomy paved the way to the development of multiple options for autologous and implant-based IBR. Skin-sparing mastectomy has been demonstrated in single institution studies to be associated with equivalent local, regional, and systemic recurrence rates as total mastectomy without reconstruction30–32. In the current study, race/ethnicity and geographic region had a particularly strong impact on rates of IBR, suggesting that there are still barriers to access for post-mastectomy breast reconstruction. The evaluation of the SEER database performed by Agarwal et al. demonstrated a similar trend correlating IBR with demographics in 2002. However, it could also be explained by the higher likelihood of diagnosis at more advanced stage disease. Unlike Agarwal et al.’s paper, which focused on all stages of breast cancer and emphasized analyses of income and demographic variables, our study sought to look at the trends in IBR for patients with newly diagnosed Stage I-III breast cancer, in the context of relevant covariates.
During much of the time period of this study, post-mastectomy XRT was used in patients with ≥4 positive lymph nodes or cancers >5 cm. This treatment pattern probably accounted for the observed similar rates of IBR in node-negative patients and those with 1–4 positive lymph nodes, and for the higher rates in node-negative patients than in those with ≥5 positive nodes. It most likely also accounted for the observed decreasing use of IBR as tumor size increased. The current study also revealed an increased use of IBR for Stage III patients when compared to previous SEER-based studies24. This trend was probably influenced by published reports, such as one in which XRT did not increase complication rates with IBR or one in which the indications for IBR were expanded to include patients with locally advanced disease who had good responses to neoadjuvant chemotherapy33,34. The relatively small but statistically significantly difference in IBR rates for ER+/PR+ (16.2%) versus ER−/PR− (14.5%) patients may reflect clinical factors not collected in the SEER database, such as the increased utilization of systemic chemotherapy in ER− patients.
Differences in rates of IBR for patients having (14%) versus not having (16.4%) post-mastectomy XRT, although statistically significant, were of a smaller magnitude than would be expected4,21,25. A survey conducted by the American Society of Plastic Surgeons in 2010 revealed that 81% of surgeons did not perform IBR in patients requiring post-mastectomy XRT19. However, trends in IBR reflect increased use of these techniques despite the receipt of adjuvant radiation. This increase in IBR has been speculated to occur for a number of reasons. Improvements in delivery mechanisms for radiation in the presence of both implant and autologous IBR coupled with patient demand for IBR, have allowed surgeons and radiation oncologists to create innovative mechanisms of offering the benefits of IBR to a wider patient population than previously reflected in the literature. These types of innovations could have also accounted for the small but statistically significant 2.4% difference in IBR rates in patients who did versus did not receive post-mastectomy XRT. It should be noted that patients who had a prior history of any cancer were left out of this analysis, thus excluding those who had been previously treated with breast conservation surgery and radiation, as well as those who had any previous cancer-directed treatment.
Our study was limited by the fact that the SEER database did not include information regarding delayed reconstruction, chemotherapy, endocrine therapy, trastuzumab, or other factors that may have influenced the surgical decision-making process including co-morbidities, smoking, obesity, previous abdominal operations, or patient preferences. Nonetheless, our study provided updated information regarding trends in the use of IBR in the United States using a large, prospective, contemporary database that represents a cross section of current breast cancer treatment in the United States. Furthermore, while our study demonstrated increased rates of IBR since 1998, it is unlikely that the Women’s Health and Cancer Rights Act of 1998 was the sole cause of this change in practice. For example, our study does not control for the variety of payers or other socioeconomic variables that may have confounded access to IBR, nor does our study control for the effect of patient co-morbidities on decision making for IBR. In addition, while our study may have suggested regional differences in rates of IBR, we were unable to determine whether these differences reflected variability in access to reconstructive surgeons versus differing viewpoints on appropriate indications for immediate post-mastectomy reconstruction.
CONCLUSIONS
The utilization of IBR for newly diagnosed breast cancer patients has increased significantly over the last decade. Using multivariate analysis, IBR was found to be significantly associated with age, race, geographical region, stage, ER, grade, LN status, and XRT (p<0.0001). Differences in rates of IBR for patients having versus not having post-mastectomy XRT were of a smaller magnitude than would be expected but were statistically significantly different. Several potentially confounding treatment variables, such as use of adjuvant or neoadjuvant chemotherapy, patient preferences, type of insurance and availability of reconstructive surgeons in the regions studied cannot be quantified using the SEER database. Nonetheless, this study is the first SEER analysis to document that rates of IBR have increased since passage of the Women’s Health and Cancer Rights Act of 1998. However, less than one in four mastectomy patients in this SEER study cohort received immediate breast reconstruction. Given advances in reconstructive techniques and acceptance of incorporating IBR into the multidisciplinary care of breast cancer patients, further increases in rates of immediate breast reconstruction are likely to be noted going forward as indications for IBR appear to be evolving.
Acknowledgement
The authors take full responsibility for the content of the paper but thank Mary Knatterud, Ph.D. (supported by the Department of Surgery of the University of Arizona), for her copyediting.
REFERENCES
- 1.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA: a cancer journal for clinicians. 2011;61:409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA: a cancer journal for clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Lee C, Sunu C, Pignone M. Patient-reported outcomes of breast reconstruction after mastectomy: a systematic review. Journal of the American College of Surgeons. 2009;209:123–133. doi: 10.1016/j.jamcollsurg.2009.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal S, Liu JH, Crisera CA, et al. Survival in breast cancer patients undergoing immediate breast reconstruction. The breast journal. 2010;16:503–509. doi: 10.1111/j.1524-4741.2010.00958.x. [DOI] [PubMed] [Google Scholar]
- 5.Petit JY, Gentilini O, Rotmensz N, et al. Oncological results of immediate breast reconstruction: long term follow-up of a large series at a single institution. Breast cancer research and treatment. 2008;112:545–549. doi: 10.1007/s10549-008-9891-x. [DOI] [PubMed] [Google Scholar]
- 6.Gieni M, Avram R, Dickson L, et al. Local breast cancer recurrence after mastectomy and immediate breast reconstruction for invasive cancer: A meta-analysis. Breast. 2012 doi: 10.1016/j.breast.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Langstein HN, Cheng MH, Singletary SE, et al. Breast cancer recurrence after immediate reconstruction: patterns and significance. Plastic and reconstructive surgery. 2003;111:712–720. doi: 10.1097/01.PRS.0000041441.42563.95. discussion 721-712. [DOI] [PubMed] [Google Scholar]
- 8.Nedumpara T, Jonker L, Williams MR. Impact of immediate breast reconstruction on breast cancer recurrence and survival. Breast. 2011;20:437–443. doi: 10.1016/j.breast.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Noone RB, Frazier TG, Noone GC, et al. Recurrence of breast carcinoma following immediate reconstruction: a 13-year review. Plastic and reconstructive surgery. 1994;93:96–106. discussion 107–108. [PubMed] [Google Scholar]
- 10.Sullivan SR, Fletcher DR, Isom CD, Isik FF. True incidence of all complications following immediate and delayed breast reconstruction. Plastic and reconstructive surgery. 2008;122:19–28. doi: 10.1097/PRS.0b013e3181774267. [DOI] [PubMed] [Google Scholar]
- 11.Zhong T, Hofer SO, McCready DR, et al. A comparison of surgical complications between immediate breast reconstruction and mastectomy: the impact on delivery of chemotherapy--an analysis of 391 procedures. Annals of surgical oncology. 2012;19:560–566. doi: 10.1245/s10434-011-1950-6. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen TJ, Costa MA, Vidar EN, et al. Effect of immediate reconstruction on postmastectomy surgical site infection. Annals of surgery. 2012;256:326–333. doi: 10.1097/SLA.0b013e3182602bb7. [DOI] [PubMed] [Google Scholar]
- 13.Schechter NR, Strom EA, Perkins GH, et al. Immediate breast reconstruction can impact postmastectomy irradiation. American journal of clinical oncology. 2005;28:485–494. doi: 10.1097/01.coc.0000170582.38634.b6. [DOI] [PubMed] [Google Scholar]
- 14.Rey P, Martinelli G, Petit JY, et al. Immediate breast reconstruction and high-dose chemotherapy. Annals of plastic surgery. 2005;55:250–254. doi: 10.1097/01.sap.0000174762.36678.7c. [DOI] [PubMed] [Google Scholar]
- 15.Warren Peled A, Itakura K, Foster RD, et al. Impact of chemotherapy on postoperative complications after mastectomy and immediate breast reconstruction. Arch Surg. 2010;145:880–885. doi: 10.1001/archsurg.2010.163. [DOI] [PubMed] [Google Scholar]
- 16.Chevray PM. Timing of breast reconstruction: immediate versus delayed. Cancer J. 2008;14:223–229. doi: 10.1097/PPO.0b013e3181824e37. [DOI] [PubMed] [Google Scholar]
- 17.Christante D, Pommier SJ, Diggs BS, et al. Using complications associated with postmastectomy radiation and immediate breast reconstruction to improve surgical decision making. Arch Surg. 2010;145:873–878. doi: 10.1001/archsurg.2010.170. [DOI] [PubMed] [Google Scholar]
- 18.Gurunluoglu R, Gurunluoglu A, Williams SA, Tebockhorst S. Current Trends in Breast Reconstruction: Survey of American Society of Plastic Surgeons 2010. Annals of plastic surgery. 2011 doi: 10.1097/SAP.0b013e31822ed5ce. [DOI] [PubMed] [Google Scholar]
- 19.Alderman AK, Hawley ST, Waljee J, et al. Understanding the impact of breast reconstruction on the surgical decision-making process for breast cancer. Cancer. 2008;112:489–494. doi: 10.1002/cncr.23214. [DOI] [PubMed] [Google Scholar]
- 20.Alderman AK, McMahon L, Jr, Wilkins EG. The national utilization of immediate and early delayed breast reconstruction and the effect of sociodemographic factors. Plastic and reconstructive surgery. 2003;111:695–703. doi: 10.1097/01.PRS.0000041438.50018.02. discussion 704-695. [DOI] [PubMed] [Google Scholar]
- 21.Chang DW, Reece GP, Wang B, et al. Effect of smoking on complications in patients undergoing free TRAM flap breast reconstruction. Plastic and reconstructive surgery. 2000;105:2374–2380. doi: 10.1097/00006534-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Alderman AK, Hawley ST, Janz NK, et al. Racial and ethnic disparities in the use of postmastectomy breast reconstruction: results from a population- based study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5325–5330. doi: 10.1200/JCO.2009.22.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joslyn SA. Patterns of care for immediate and early delayed breast reconstruction following mastectomy. Plastic and reconstructive surgery. 2005;115:1289–1296. doi: 10.1097/01.prs.0000156974.69184.5e. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal S, Pappas L, Neumayer L, Agarwal J. An analysis of immediate postmastectomy breast reconstruction frequency using the surveillance, epidemiology, and end results database. The breast journal. 2011;17:352–358. doi: 10.1111/j.1524-4741.2011.01105.x. [DOI] [PubMed] [Google Scholar]
- 25.Alderman AK, Wei Y, Birkmeyer JD. Use of breast reconstruction after mastectomy following the Women's Health and Cancer Rights Act. JAMA : the journal of the American Medical Association. 2006;295:387–388. doi: 10.1001/jama.295.4.387. [DOI] [PubMed] [Google Scholar]
- 26.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plastic and reconstructive surgery. 2013;131:15–23. doi: 10.1097/PRS.0b013e3182729cde. [DOI] [PubMed] [Google Scholar]
- 27.Christian CK, Niland J, Edge SB, et al. A multi-institutional analysis of the socioeconomic determinants of breast reconstruction: a study of the National Comprehensive Cancer Network. Annals of surgery. 2006;243:241–249. doi: 10.1097/01.sla.0000197738.63512.23. [DOI] [PMC free article] [PubMed] [Google Scholar]