Abstract
We have developed a calcium phosphate glass (CPG) doped with Zn2+ or F− or combined Zn2+ and F− ions, which are naturally found in the human body and play a dual role in bone formation and antibacterial activity. Previously, we have demonstrated that this family of CPGs has superior osteoconductive and resorbable properties in vivo. This study aimed to investigate the antibacterial property of CPGs incorporating Zn2+ and/or F−. We used Streptococcus mutans as a model organism because it is one of the major human oral pathogens and an early colonizer, and it has been associated with several oral infections, such as dental caries, periodontitis, and peri-implantitis. 0.01g and 0.05g of CPGs were incubated with Streptococcus mutans for 0, 2, 4, and 6 h. Serial dilutions were plated in triplicate and colony forming units were determined. The antimicrobial effect of CPG incorporating Zn2+ or F− was greater than CPG incorporating both these ions. CPG without doping produced a moderate antimicrobial effect. This family of CPGs, previously shown to promote new bone formation in vivo, is demonstrated to have superior bactericidal properties.
Keywords: Calcium phosphate glass, antimicrobial properties, Streptococcus mutans, zinc, fluoride, phosphate
1. INTRODUCTION
Bioactive glasses, especially phosphate-based glasses have been the focus of much interest in recent years, due to their bioactive nature and ability to promote generation of soft and hard tissue.1–4 The ionic dissolution products support cell proliferation, cell differentiation and activate gene expression.5 The biological response to artificial materials depends on parameters such as chemical composition, topography, porosity and grain size.6 By varying the chemical composition of glass constituents, the biological attributes of bioactive glasses can be tuned, to combat bacterial associated infections, and facilitate tailorability for specific medical applications.3 Bacterial infections hinder the bone healing process, and at times, lead to surgical failures.7 Hence, the integration of certain metal ions to the bioactive glasses can function as therapeutic for various pathological conditions associated with bacterial colonization and infection. The phosphate-based glasses when doped with appropriate metal ions can help fight infections as a controlled release antibacterial delivery system.4,8–10 The unique feature of these glasses is the release of integrated ions upon implantation or exposure to physiological environment and formation of hydroxy-carbonate apatite layer that bonds directly to bone.5,11–13
In this study, we focused on ions that are found in the human body and are able to play a dual role in bone formation and antibacterial activity. Ions that fall in this category are fluoride (F−), magnesium (Mg2+), zinc (Zn2+) and phosphate (PO4+).
Fluoride is known to disrupt bacterial enzymes and membrane function.14 It can inhibit the glycolytic enzyme, enolase, in S. mutans in the presence of magnesium and phosphate ions.15 Moreover, fluoride has been shown to have a two-fold acidifying effect on the cytoplasms of S. mutans: one, through inhibiting the transmembrane protein, F-ATPase, which counters cytoplasmic pH changes; and two, by crossing the cell membrane in the form of hydrofluoric acid (HF) in low pH (< 4) environments, which quickly dissociates to become H+ and F− ions.16,17 Through this combined action, concentrations as low as 0.1 mM of fluoride have been found to prevent glycolysis in S. mutans.
Previous studies have demonstrated that zinc expedites hard tissue formation by decreasing osteoclast activity while also increasing rates of osteoblast adhesion and proliferation.18,19 In in vivo studies, Kawamura et al20 and Ito et al21 have demonstrated that zinc promotes bone formation when incorporated in conjunction with calcium phosphate materials (β-TCP and β-TCP/HA composite). Boyd et al22 has reported that 1 ppm levels of released zinc from glass cements were effective in inhibiting S. mutans growth. Atmaca et al found that even lower levels, 0.02 ppm solutions of zinc acetate, were capable of suppressing growth of planktonic Streptococcus auerus and Staphylococcus epidermidis, two strains of virulent, Gram-positive bacteria.23 Several studies have supported these results by demonstrating that in the presence of < 1 mM of Zn2+, lactic acid production in S. mutans, Streptococcus sobrinus and Streptococcus salivarius was significantly reduced.21,24,25
Phosphate is widely accepted as a fundamental building block for most organisms and therefore few studies have investigated the effect of super-physiological concentrations of phosphate on microbial growth and viability. However, in the cases where phosphate and pH levels have been carefully monitored in relation to microbial inhibition, a controversial theory has begun to take shape, where phosphate ions alone can be either bacteriostatic or bactericidal.26–28
Previously, we have demonstrated that a novel calcium phosphate glass, part of the CaO-P2O5-NaF-MgO-ZnO system, has osteoconductive and resorbable characteristics in vivo.29,30 In particular, studies by Lee et al30 have demonstrated that glasses with a Ca/P molar ratio of 0.6 increased mineralization and alkaline phosphatase activity of MC3T3 cells, a preosteoblast-like cell line. Additionally, the same calcium phosphate glass composition was shown to promote almost complete bony in-growth in critical size calvarial defects in rats by 9 weeks.31 However, no antimicrobial assays have been performed, despite the known beneficial effects of zinc and fluoride. This study focuses on characterization of novel calcium phosphate glass compositions doped with zinc and/or fluoride and evaluates their antibacterial properties on the viability of Streptococcus mutans. We chose Streptococcus mutans as a model organism because it is one of the major human oral pathogens and an early colonizer, and it has been associated with several oral infections, such as dental caries, periodontitis, and peri-implantitis.32–39
2. MATERIALS & METHODS
2.1. Preparation of calcium phosphate glass
The experimental material compositions (Ca to P ratio and the percentage of doping with MgO, ZnO and NaF) were modeled after the bioactive glass formulation examined by LeGeros and Lee.40
The base calcium phosphate glass (CPG) was prepared from a mixture of CaCO3, H3PO4 and MgO with Ca:P molar ratio of 0.6. The mixture was dried at 80°C for 2 h (Fisher Scientific Isotemp 500 Series; Thermo Fisher Scientific, Waltham, MA, USA). The dried powder was placed into a platinum crucible and calcined at 850°C for 2 h (Barnstead Thermolyne 4800; Thermo Fisher Scientific) to remove volatile impurities. Final melting of the glass was achieved at 1200°C for 2 h (Barnstead Thermolyne 4800; Thermo Fisher Scientific). Quenching the melted glass into ice water formed glass frits. Then, the frits were ground into a fine powder using an alumina mortar and pestle. The experimental groups, CPGs doped with Zn2+ (CPG+Zn), F− (CPG+F), or combined Zn2+ and F− (CPG+Zn+F) were prepared using the same procedure as described above. The addition of 1 wt% ZnO and 0.5 wt% NaF, were added to the initial mixture of CaCO3, H3PO4, and MgO for CPG+Zn formulations and CPG+F formulations, respectively. CPG+Zn+F is doped with both ZnO and NaF.
All glass compositions were sieved between 60 µm mesh and 250 µm mesh before sterilization by autoclaving (dry cycle) at 121°C for 15 min.
2.2. Material characterization
Final compositions of the glasses with Ca:P molar ratio of 0.6 were detected using x-ray fluorescence (XRF) analysis (n = 3). The crystal structure of the glasses was determined using an x-ray diffractometer (XRD) (Philips X’Pert, Philips Analytical Inc., MA, USA) with a curved crystal monochromator and copper Kα radiation (n = 2). The voltage and current were set at 45 kV and 45 mA, respectively. The scanning range spanned between 20° – 40° 2θ with a step size of 0.02° and a dwell time of 3 s/step.
Dissolution studies (n = 3), which determined the release of calcium, phosphorus, zinc and magnesium ions, were performed using an inductively coupled plasma spectroscopy (ICP) (Thermo Jarrell Ash, Franklin, MA, USA). 0.05 g of glass particulate was placed into 25 ml of potassium acetate solution (pH = 6) and kept in a 37°C water bath. The characteristic wavelengths selected were 317.9 Å (Ca), 279.6 Å (Mg), 213.6 Å (P), and 202.5 Å (Zn). Six measurements per wavelength were taken every 3 min with a total duration of 4 h for each sample. An ion selective electrode (Orion, Waltham, MA, USA) attached to a pH Stat system (Metrohm Brinkmann, Delran, NJ, USA) was used to measure the release of fluoride ions (n = 3). A four-point calibration curve with a R2 value > 0.99 was used to calculate fluoride concentration as a function of time.
The pH of the CPGs incubated in phosphate buffer saline (PBS) was measured in order to gauge the ionic environment during bacterial viability studies. In order to compensate for the size of the pH meter probe (Metrohm 692 pH/Ion Meter, Metrohm, Riverview, FL, USA), a higher volume of buffer (1 ml) was necessary to cover the sensor. Therefore, a scaled up version of the bacterial studies was made so that the solid to liquid ratio (1:1) remained the same. pH values (n = 3) were allowed to equilibrate to a final value after ten min and then monitored over the course of an hour.
2.3. Effect of bioactive glass on S. mutans
S. mutans UA 159 was maintained on tryptic soy agar (Fisher Scientific, Pittsburg, PA, USA) for 48 h at 37°C under anaerobic conditions (5% CO2). The cultures (n = 2) were grown overnight under the same conditions in brain heart infusion broth (Fisher Scientific, USA) and the late log phase cultures were harvested at the cell concentration of 106 – 107 CFU/ml with an approximate optical density (OD) of 0.4. The bacterial concentrations were measured with a Biomate spectrophotometer (Thermo Electron Corporation, Waltham, MA, USA) at 660 nm.
0.01 g and 0.05 g of CPGs were weighed into separate 1.5 mL cryotubes (USA Scientific, Orlando, FL, USA) for each time point. A negative control tube contained no particulates. To each tube, 50 µL of S. mutans culture was added and tubes were incubated under anaerobic conditions (5% CO2) for 0, 2, 4, or 6 h at 37°C. The time points were selected based on Allen et al’s study41 indicating 80% killing of S. mutans incubated with Perioglas® for 3 h. At the specified time interval, 950 µL of PBS buffer was added to all test tubes to bring the total volume to 1 ml. All samples were then vortexed for 30 sec. Eight 10-fold serial dilutions in PBS were prepared and 100 µL of each were plated on TSA in triplicate (n = 3). The plates were incubated for 48 h in a 5% CO2 incubator (NAPCO Series, Thermo Fisher Scientific) at 37°C. Quadruplicate experiments were performed and the results were averaged.
2.4. Statistical analysis
Averaged results of multiple experiments were expressed as mean ± standard deviation. The data was subjected to three-way analysis of variance (ANOVA) to determine any significant differences between the different CPG compositions with a p < 0.05 indicating significant difference.
3. RESULTS
3.1. Compositional and chemical properties of CPGs
Using XRF analysis, weight percent oxide composition of the formed calcium phosphate glasses is shown in Table 1. All constituents were determined except for fluoride (atomic number, Z = 9), due to the XRF detection limits of Z = 11, or sodium. The results are in line with our intended compositions.
Table 1.
Weight percent composition of various CPGs with an intended Ca/P molar ratio of 0.6 as analyzed by XRF.
| Base CPG | CPG+Zn | CPG+F | CPG+Zn+F | |
|---|---|---|---|---|
| Analyzed Compounds (wt%) | ||||
| CaO | 36.4 ± 0.5 | 36.0 ± 0.5 | 37.7 ± 0.7 | 36.5 ± 0.5 |
| MgO | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 |
| Na2O | - | - | 1.3 | 1.0 |
| P2O5 | 62.3± 0.5 | 61.7± 0.5 | 59.7± 0.5 | 60.2± 0.5 |
| ZnO | - | 1.2 ± 0.1 | - | 1.2 ± 0.1 |
| Calculated Ca/P | 0.58 | 0.58 | 0.63 | 0.61 |
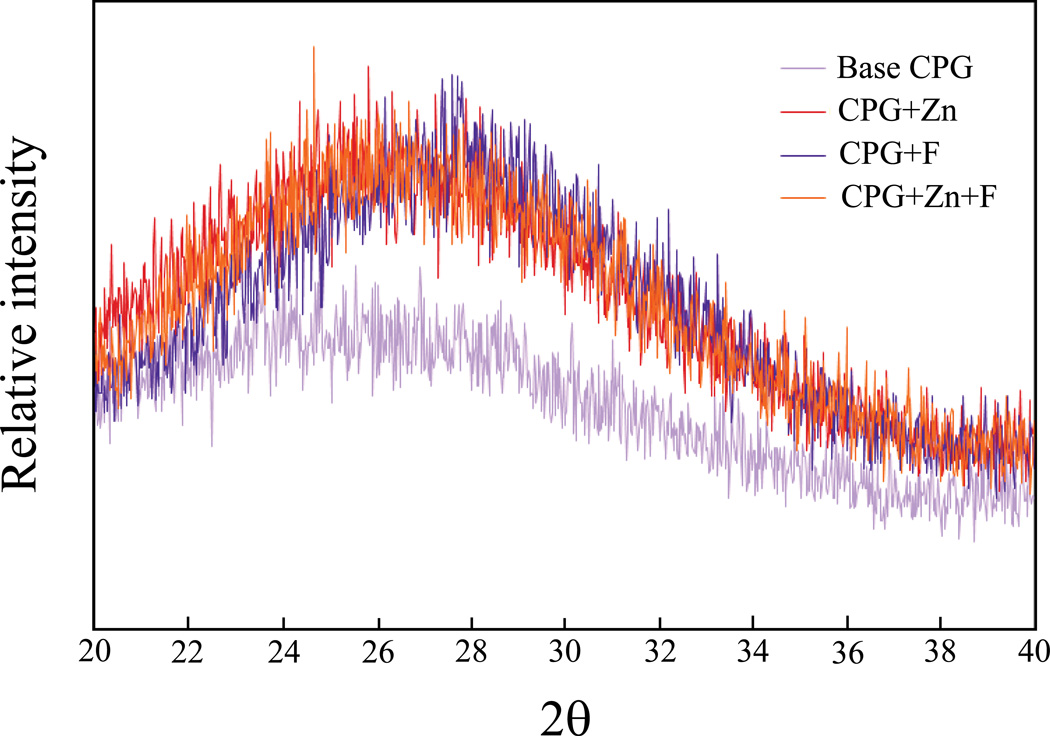
XRD patterns of Ca/P glasses are shown in Fig 1. All glasses demonstrated a lack of defined peaks between 20° – 40° 2θ, which characterizes long-range disorder within the structure. The addition of supplemental ion components (Zn2+ and F−), produced an XRD pattern with a slightly narrower amorphous hump than that of base CPG.
Figure 1.
Comparison of XRD spectra among calcium phosphate glasses.
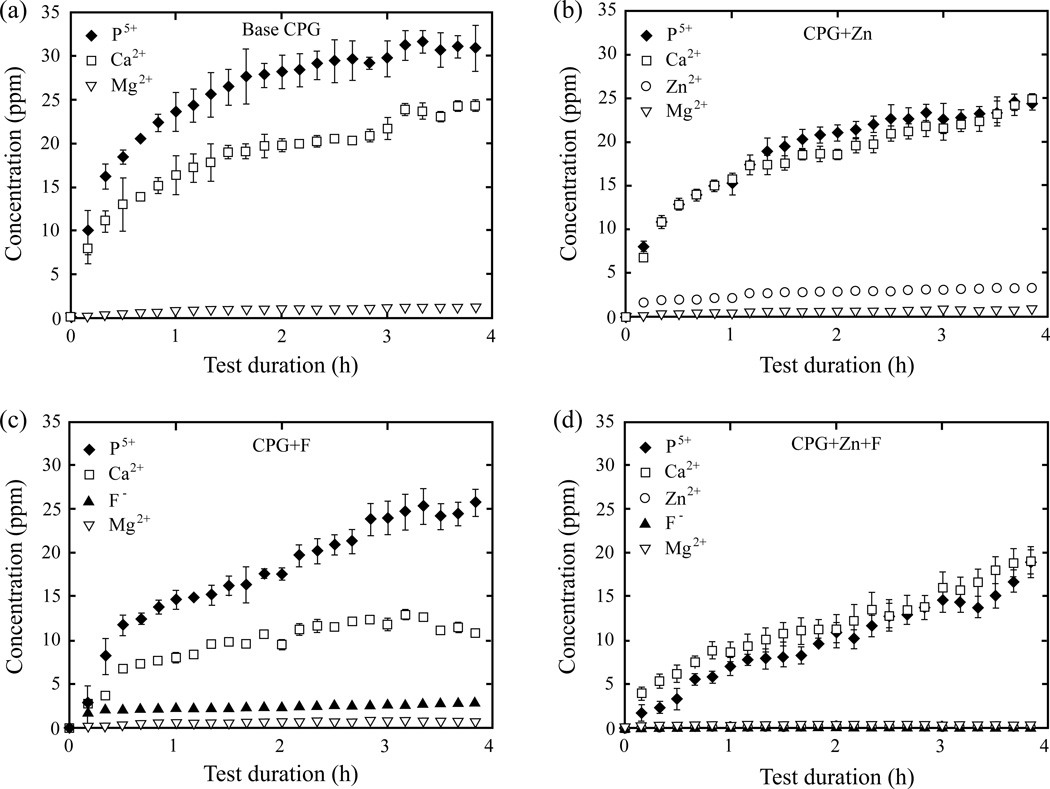
The ion release profile of CPGs over the course of 4 h is shown in Fig. 2. The total concentrations of released ions are shown in Table 2. The solubility of CPGs decreased when doped with zinc and fluoride. The total P5+ ion concentration dropped significantly from 30.9 ± 2.64 ppm in the base CPG to 24.4 ± 1.11 ppm in CPG+Zn and 25.7 ± 1.54 ppm in CPG+F to 18.9 ± 1.93 ppm in CPG+Zn+F. On the other hand, calcium ion release was unaffected by the addition of zinc (25.1 ± 0.52 ppm) to the base CPG (24.3 ± 0.64 ppm). CPG+F released the least Ca2+ (10.9 ± 0.40 ppm) among the four CPG compositions, since it was observed that CPG+Zn+F (19.1 ± 1.50 ppm) was able to regain some degree of calcium release. Magnesium release was highest in the base CPG (1.04 ± 0.06 ppm) and least in CPG+Zn+F (0.09 ± 0.01 ppm). Additionally, fluoride alone reduced the magnesium release more than zinc alone, showing 0.67 ± 0.03 ppm and 0.79 ± 0.02 ppm, respectively. The combined influence of fluoride and zinc made the most impact on CPG solubility for Zn and F ions, reducing total released Zn2+ from 3.33 ± 0.01 ppm to 0.12 ± 0.01 ppm and F− from 2.88 ± 0.03 ppm to 0.03 ± 0.002 ppm.
Figure 2.
Rate of CPG dissolution with respect to time.
Table 2.
Concentration in ppm of released ions after 4 h for CPGs with a Ca/P molar ratio of 0.6.
| Ions | Base CPG | CPG+Zn | CPG+F | CPG+Zn+F |
|---|---|---|---|---|
| P5+ | 30.9 ± 2.64 | 24.4 ± 1.11 | 25.7 ± 1.54 | 18.9 ± 1.93 |
| Ca2+ | 24.3 ± 0.64 | 25.1 ± 0.52 | 10.9 ± 0.40 | 19.1 ± 1.50 |
| Mg2+ | 1.04 ± 0.06 | 0.79 ± 0.02 | 0.67 ± 0.03 | 0.09 ± 0.01 |
| Zn2+ | 0 | 3.33 ± 0.13 | 0 | 0.12 ± 0.01 |
| F− | 0 | 0 | 2.88 ± 0.03 | 0.03 ± 0.002 |
In order to facilitate direct comparisons with previous studies, ppm values were translated to molarity concentrations. This was achieved using the formula:
| (1) |
where [I] is the ionic concentration (in ppm) as obtained through ICP, MW represents the molecular weight of each ion, and 500 is the volumetric proportionality conversion (25 ml/0.05 ml). Translated values of Table 2 are shown in Table 3.
Table 3.
Calculated Min-vitro of released ions from CPGs with a Ca/P molar ratio of 0.6 after 4 h using eq. 1.
| Ions | Base CPG | CPG+Zn | CPG+F | CPG+Zn+F |
|---|---|---|---|---|
| P5+ | 0.50 ± 0.04 | 0.39 ± 0.02 | 0.41 ± 0.02 | 0.31 ± 0.03 |
| Ca2+ | 0.30 ± 0.01 | 0.31± 0.01 | 0.14 ± 0.001 | 0.24 ± 0.01 |
| Mg2+ | 0.02 ± 0.001 | 0.02 ± 0.001 | 0.01 ± 0.001 | < 0.01 |
| Zn2+ | 0 | 0.03 ± 0.001 | 0 | < 0.01 |
| F− | 0 | 0 | 0.08 ± 0.001 | < 0.01 |
The addition of zinc and fluoride, alone or in combination, caused a decrease in the overall solubility of the CPGs. However, incorporation of either zinc or fluoride alone, produced CPGs that released higher than reported MIC (minimum inhibitory concentration) values for S. mutans.42–44 This result was not observed when the ions were doped in combination. In fact, the solubility of the doped CPG was significantly reduced with only parts per billion of zinc and fluoride ions detectable.
When immersed in PBS, CPGs (with or without doping) maintained a slightly acidic pH (~ 6).
3.2. Effect of CPGs on S. mutans
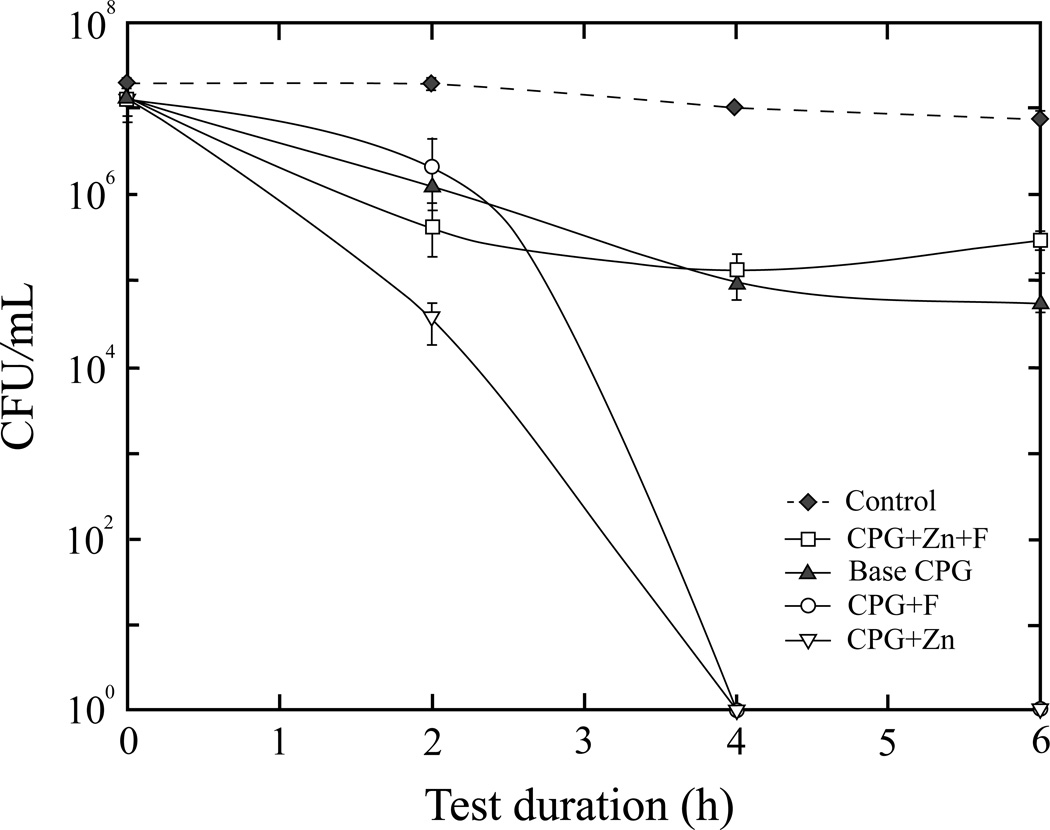
At 0.01 g (Fig. 3), CPG+Zn caused the most reduction in viability at 2 h (3.68 × 104 CFU/ml). At 4 h, CPG+Zn and CPG+F both showed 100% bactericidal activity that continued for the length of the study. In the presence of CPG+Zn+F and base CPG at 6 h, viable counts decreased from 1.31 × 107 to 3.09 × 105 and 5.48 × 104/ml, respectively. Total viable load (CFU/ml) of S. mutans in the negative control (contained no glass particulates) remained almost unchanged throughout the 6 h test duration.
Figure 3.
Viable S. mutans counts after exposure to 0.01 g of CPGs, or negative control (no glass).
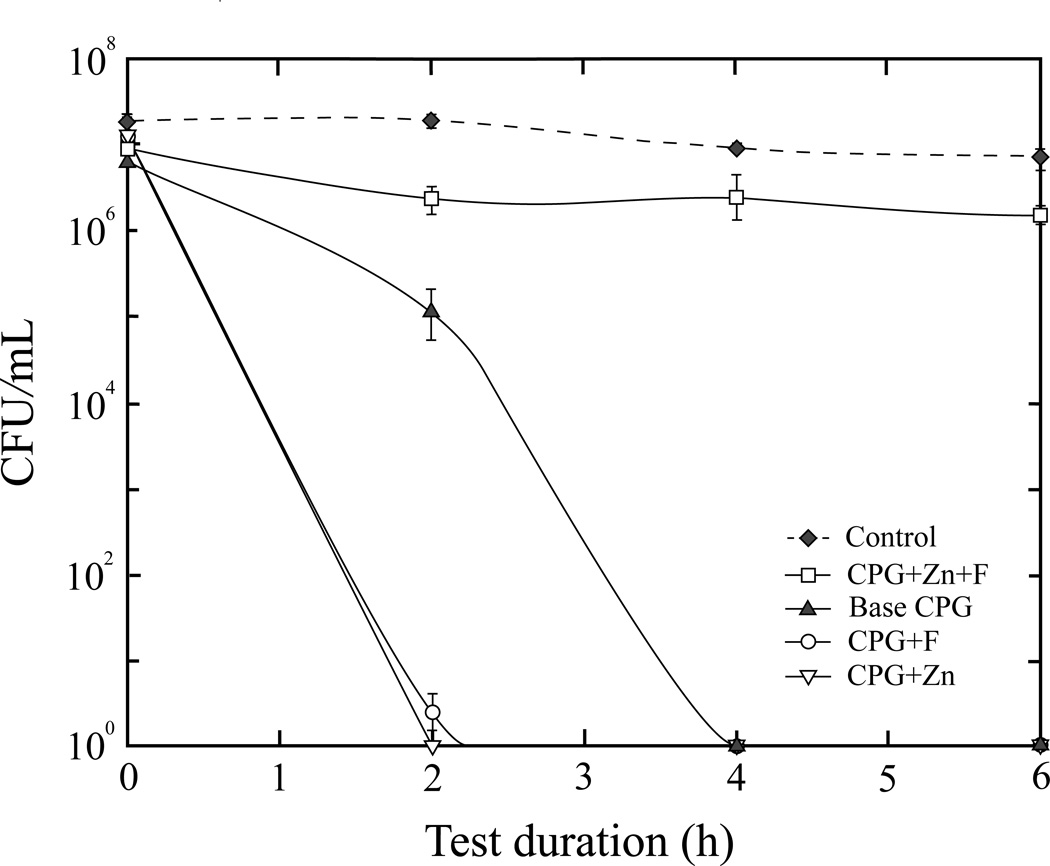
Increasing the concentration of CPG+Zn and CPG+F to 0.05 g (Fig. 4) produced a maximum bactericidal effect at 2 h compared to 4 h with 0.01 g. The base CPG at 0.05 g also had a higher antimicrobial efficacy, with complete inhibition occurring at 4 h. The activity of CPG+Zn+F remained unchanged regardless of concentration. There was no noticeable decrease in viable counts for the negative control.
Figure 4.
Viable S. mutans counts after exposure to 0.05 g of CPGs, or negative control (no glass).
4. DISCUSSION
In this study, we fabricated a series of CPG compositions in order to evaluate the effect of zinc, fluoride, or zinc and fluoride doping on S. mutans activity. Despite the similarities among the CPG compositions, there were differences in solubility and the effect of solubilized ions that contributed to each material’s unique antimicrobial activity. Antibacterial activity was also found to be dependent on solid loading, with 0.05 g showing a greater effect relative to 0.01 g. Interestingly, the bactericidal effect of zinc and fluoride ions was greatest when they were incorporated separately in the CPGs. CPG incorporating the combination of zinc and fluoride ions (CPG+Zn+F) demonstrated higher crystallinity, lower solubility, and thus lower killing.
Since the effect of solubilized ions was central to our study, we carefully analyzed the concentrations of the individual ions released as a function of time using ICP. It is important to note that the concentration of solubilized ions that was measured using ICP is not equivalent to concentrations seen in vitro due to volumetric differences (25 ml of solution in ICP vs. 50 µL of solution in vitro) as necessitated by the parameters of each experiment.
Adding either fluoride or zinc was able to increase killing rates compared to the base CPG. Furthermore, at 0.01 g, CPG+Zn demonstrated a significantly lower decrease in CFUs at 2 h than CPG+F. These findings may be attributed to zinc’s higher uptake into bacterial cells at neutral pHs, where it acts to inhibit ATP synthesis, whereas fluoride is most effective at acidifying cytoplasms at pH values below 5.45 Additionally, the activities of fluoride and zinc have been shown not only to have antimicrobial effects for planktonic cells, but significant effects on single-species biofilms as well.25
Although several studies have shown data supporting the additive effect of zinc and fluoride on the inhibition of microbial metabolic functions, this was not the case with our investigation. CPG+Zn+F produced the least amount of killing. This result is mainly because of differences in the form of zinc and fluoride ions that are being introduced. Previous studies have used aqueous ionic solutions in order to introduce Zn2+ and F−, thereby making the antimicrobial agents freely available.14,46,47 In our case, the ions are incorporated into the glass structure as network modifiers, and thus suppress their own release into the extracellular environment. This is especially true of Zn2+ and F− co-doped CPGs, where minimal dissolution was detected. Similar results have been documented in other studies of bioactive glasses. Haimi et al45 studied glasses in the family of Na2O–K2O–MgO–CaO–P2O5–B2O3–TiO2–SiO2–ZnO, with zinc oxide doping from 0 – 5 mol%. Their results showed decreasing solubility profiles as zinc concentrations increased in the glass composition. Again, no change in crystallinity was observed, even at the highest doping. Even low concentrations of zinc doping (0.1 wt%) caused an appreciably slower dissolution rate when compared to non-doped Bioglass®48. This supports zinc’s active role as a glass network modifier, stabilizing non-bridging oxygen bonds. On the other hand, fluoride has been known to partially substitute into the crystalline lattice of calcium phosphate (principally, apatite) materials and decrease solubility through stabilizing effects.49–51
Generally, slower dissolution rates are advantageous for bioactivity since they allow for material resorption to be balanced with tissue regeneration. It also allows for a more prolonged release of antimicrobial agents. At the same time, we must remember that over-stabilization of the CPG network can cause ionic dissolution to be dampened to the point of being ineffective. Therefore, when considering a biologically active dopant for CPGs, the structural functionality of these components must also be considered.
Despite the absence of bactericidal additives zinc and fluoride, the base CPG still exhibited some antibacterial properties (Fig. 4). The ions released from the base CPG are calcium, phosphorous, and a trace amount of magnesium (Table 2). The Mg2+ ion has been reported to have a bactericidal effect, provided its ion concentration is above the threshold ~104 ppm.52 The amount of Mg2+ ions that are released by base CPG is around 1 ppm. Therefore, it is highly unlikely that the antibacterial property of base CPG is due to this trace concentration of Mg2+ ions. Indeed, many bacteria, including S. mutans, are often suspended in solutions such as Dulbecco's PBS and Butterfield's Phosphate Diluent which contain millimolar amounts of MgCl2 (on the order of several tens of ppm) and are known to have no detrimental effect on bacterial activities.
Ca2+ ions released from our base CPG is around 0.30 M. A previous study suggested that at this concentration Ca2+ might have inhibitory effect on S. mutans.53 However, our CPG+Zn+F also produced similar amount of Ca2+ (0.24 M, Table 2) yet exhibited little bactericidal effect (Fig. 4), suggesting that the antibacterial property of base CPG was most likely not attributed to Ca2+. Therefore, the only ion that may have antibacterial effect in our base CPG is phosphate.
Although this may be surprising in terms of the essential role that phosphate plays in basic cell functions and metabolism, it is critical to note that the level of phosphate is a determining factor in whether it facilitates or inhibits microbial growth. Generally, phosphate concentrations recommended for microbial growth culture range from 2 – 10 ppb; conversely, the phosphate levels released by our CPGs are 103 times greater (Table 2).54 Therefore, although not commonly recognized as an antimicrobial agent, certain parameters do allow phosphate to inhibit microbial growth and viability. For example, previous studies by Moreau et al55 showed inhibition of S. mutans from spray-dried amorphous calcium phosphate nanoparticles. However, their study did not evaluate the concentration of eluted ions. More specifically then, Handelman et al56 observed that at pH ≥ 7, adding 0.06 M of phosphate ions to S. mutans liquid cultures resulted in significantly lower lactic acid production. At pH 6.5, there was minimal effect on acid production, even with increasing phosphate concentrations up to 0.1 M. In our study, the pH of our CPG containing media was around 6. When the phosphate concentration was 0.31 M (CPG+Zn+F), limited antibacterial effect was observed. But when the phosphate concentration reached 0.50 M (base CPG), bacterial killing occurred, probably due to metabolic failure. A formal mechanism for this phenomenon was proposed by Brown et al26, who demonstrated that while low phosphate concentrations (< 25 mM) promoted lactate dehydrogenase activity (LDH), a critical glycolytic enzyme which converts pyruvate into lactic acid, higher concentrations of phosphate bound the LDH activator, fructose-1,6-diphosphate (FDP), and prevented LDH activity. Therefore, through this pathway, phosphate is able to mitigate S. mutan’s lactic acid production, reducing the organism’s cariogenic abilities and metabolic function. Other forms of phosphate have been found to have bactericidal effects as well. In fact, studies have shown that polyphosphates have a broad range of growth inhibition among both Gram-positive and Gram-negative bacteria, such as: Escherichia coli, Salmonella typhimurium, Bacillus subtilis and Staphylococcus aureus57–59 Polyphosphates can serve as divalent cation chelators of crucial metals that are necessary for cell wall integrity and division.28 It has also been shown that they can act as iron chelators, making hemin unavailable to bacteria, such as Porphyromonas gingivalis, that require heme as a nutrient for growth. Although the form of phosphate ion that is released by CPGs was not evaluated in this study, it is feasible that more than one type is acting to suppress S. mutans. Due to the pyrophosphate glass structure in CPGs, both P2O74− ions and their simpler subunit orthophosphate, PO43−, may be present in solution as well as all their myriad protonation states. Therefore, there is a possibility of additive inhibitory effects through the various phosphates.
At the same time, research on synthetic carbonate apatites reveals that higher PO43− concentrations than the ones seen here are released in vivo without any toxic effects and function to stimulate bone formation.60 Therefore, high concentrations of phosphate do not cause cellular distress and, in fact, are necessary to promote precipitation of carbonate apatite for bone repair.
5. CONCLUSION
This study investigated and identified several CPGs that are able to confer both bioactivity and antimicrobial properties. Among these were CPGs that were singularly doped with the antimicrobial ions, zinc or fluoride. Our results contradicted conventional wisdom that doping materials with multiple antimicrobial agents would offer additive benefits, since CPG co-doped with zinc and fluoride demonstrated significant decreases in solubility that suppressed the amount of ions released in solution.
Results of this study demonstrated that CPGs have a high antimicrobial efficacy while maintaining a physiologically near neutral pH environment. Some of the bactericidal activity can be attributed to the release of antimicrobial ions, zinc and fluoride. Additionally, this study demonstrated the substantial antibacterial effects of high concentrations of phosphate anions at near neutral pH with a pure CaO–P2O5–MgO glass system.
ACKNOWLEDGEMENT
This study was supported by NIH/NIDCR 2R01 DE017925 (PI. Zhang); NSF/CMMI-0758530 (PI. Zhang); NIH/NIAMS R01 AR056208 (PI. LeGeros/Zhang); NIH/NIDCR DE019178 (PI. Saxena); and NIH/NIDCR DE020891 (PI. Saxena).
Footnotes
DISCLOSURES
We declare that we have no conflicts of interest.
REFERENCES
- 1.Abou Neel EA, Chrzanowski W, Knowles JC. Effect of increasing titanium dioxide content on bulk and surface properties of phosphate-based glasses. Acta Biomaterialia. 2008;4(3):523–534. doi: 10.1016/j.actbio.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Bitar M, Salih V, Mudera V, Knowles JC, Lewis MP. Soluble phosphate glasses: in vitro studies using human cells of hard and soft tissue origin. Biomaterials. 2004;25(12):2283–2292. doi: 10.1016/j.biomaterials.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 3.Ensanya AAN, David MP, Sabeel PV, Robert JN, Jonathan CK. Bioactive functional materials: a perspective on phosphate-based glasses. J Mater Chem. 2009;19(6):690–690. [Google Scholar]
- 4.Mulligan AM, Wilson M, Knowles JC. Effect of increasing silver content in phosphate-based glasses on biofilms of Streptococcus sanguis. Journal of Biomedical Materials Research Part A. 2003;67A(2):401–412. doi: 10.1002/jbm.a.10052. [DOI] [PubMed] [Google Scholar]
- 5.Murphy S, Wren AW, Towler MR, Boyd D. The effect of ionic dissolution products of Ca–Sr–Na–Zn–Si bioactive glass on in vitro cytocompatibility. Journal of Materials Science: Materials in Medicine. 2010;21(10):2827–2834. doi: 10.1007/s10856-010-4139-9. [DOI] [PubMed] [Google Scholar]
- 6.Hoppe A, Güldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32(11):2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Lim PN, Teo EY, Ho B, Tay BY, Thian ES. Effect of silver content on the antibacterial and bioactive properties of silver-substituted hydroxyapatite. Journal of Biomedical Materials Research Part A. 2013 doi: 10.1002/jbm.a.34544. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 8.Bromberg LE, Buxton DK, Friden PM. Novel periodontal drug delivery system for treatment of periodontitis. Journal of Controlled Release. 2001;71(3):251–259. doi: 10.1016/s0168-3659(01)00226-7. [DOI] [PubMed] [Google Scholar]
- 9.Franks K, Abrahams I, Georgiou G, Knowles JC. Investigation of thermal parameters and crytallisation in a ternary CaO–Na2O–P2O5-based glass system. Biomaterials. 2001;22(5):497–501. doi: 10.1016/s0142-9612(00)00207-6. [DOI] [PubMed] [Google Scholar]
- 10.Sanzana ES, Navarro M, Macule F, Suso S, Planell JA, Ginebra MP. Of the in vivo behavior of calcium phosphate cements and glasses as bone substitutes. Acta Biomaterialia. 2008;4(6):1924–1933. doi: 10.1016/j.actbio.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 11.Hench L. Bioceramics: from concept to clinic. J Am Ceram Soc. 1991;7:1487–510. [Google Scholar]
- 12.Jell G, Stevens MM. Gene activation by bioactive glasses. Journal of Materials Science: Materials in Medicine. 2006;17(11):997–1002. doi: 10.1007/s10856-006-0435-9. [DOI] [PubMed] [Google Scholar]
- 13.Moore WR, Graves SE, Bain GI. Synthetic bone graft substitutes. ANZ Journal of Surgery. 2001;71(6):354–361. [PubMed] [Google Scholar]
- 14.Marquis RE. Antimicrobial actions of fluoride for oral bacteria. Canadian Journal of Microbiology. 1995;41(11):955–964. doi: 10.1139/m95-133. [DOI] [PubMed] [Google Scholar]
- 15.Qin R, Chai GQ, Brewer JM, Lovelace LL, Lebioda L. Fluoride inhibition of enolase: Crystal structure and thermodynamics. Biochemistry. 2006;45(3):793–800. doi: 10.1021/bi051558s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sturr MG, Marquis RE. Inhibition of proton-translocating ATPases of Streptococcus-mutans and Lactobacillus-casei by fluoride and aluminum. Archives of Microbiology. 1990;155(1):22–27. doi: 10.1007/BF00291269. [DOI] [PubMed] [Google Scholar]
- 17.Norowski PA, Bumgardner JD. Biomaterial and antibiotic strategies for peri-implantitis. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2009;88B(2):530–543. doi: 10.1002/jbm.b.31152. [DOI] [PubMed] [Google Scholar]
- 18.Otsuka M, Oshinbe A, LeGeros RZ, Tokudome Y, Ito A, Otsuka K, Higuchi WI. Efficacy of the injectable calcium phosphate ceramics suspensions containing magnesium, zinc and fluoride on the bone mineral deficiency in ovariectomized rats. Journal of Pharmaceutical Sciences. 2008;97(1):421–432. doi: 10.1002/jps.21131. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa K, Miyamoto Y, Yuasa T, Ito A, Nagayama M, Suzuki K. Fabrication of Zn containing apatite cement and its initial evaluation using human osteoblastic cells. Biomaterials. 2002;23(2):423–428. doi: 10.1016/s0142-9612(01)00121-1. [DOI] [PubMed] [Google Scholar]
- 20.Kawamura H, Ito A, Miyakawa S, Layrolle P, Ojima K, Ichinose N, Tateishi T. Stimulatory effect of zinc-releasing calcium phosphate implant on bone formation in rabbit femora. Journal of Biomedical Materials Research. 2000;50(2):184–190. doi: 10.1002/(sici)1097-4636(200005)50:2<184::aid-jbm13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Ajdić D, McShan WM, McLaughlin RE, Savić G, Chang J, Carson MB, Primeaux C, Tian R, Kenton S, Jia H, et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proceedings of the National Academy of Sciences. 2002;99(22):14434–14439. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyd D, Li H, Tanner DA, Towler MR, Wall JG. The antibacterial effects of zinc ion migration from zinc-based glass polyalkenoate cements. Journal of Materials Science-Materials in Medicine. 2006;17(6):489–494. doi: 10.1007/s10856-006-8930-6. [DOI] [PubMed] [Google Scholar]
- 23.Atmaca S, Gul K, Cicek R. The effect of zinc on microbial growth. Turkish Journal of Medical Sciences. 1998(28):595–597. [Google Scholar]
- 24.Oppermann RV, Rolla G, Johansen JR, Assev S. Thiol-groups and reduced acidogenicity of dental plaque in the presence of metal-ions in vivo. Scandinavian Journal of Dental Research. 1980;88(5):389–396. doi: 10.1111/j.1600-0722.1980.tb01244.x. [DOI] [PubMed] [Google Scholar]
- 25.Phan TN, Buckner T, Sheng J, Baldeck JD, Marquis RE. Physiologic actions of zinc related to inhibition of acid and alkali production by oral streptococci in suspensions and biofilms. Oral Microbiology and Immunology. 2004;19(1):31–38. doi: 10.1046/j.0902-0055.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 26.Brown AT, Ruh R. Negative interaction of orthophosphate with glycoltic metabolism by Streptococcus mutans as a possible mechanism for dental caries reduction. Archives of Oral Biology. 1977;22(8–9):521–524. doi: 10.1016/0003-9969(77)90048-6. [DOI] [PubMed] [Google Scholar]
- 27.Maier SK, Scherer S, Loessner MJ. Long-chain polyphosphate causes cell lysis and inhibits Bacillus cereus septum formation, which is dependent on divalent cations. Applied and Environmental Microbiology. 1999;65(9):3942–3949. doi: 10.1128/aem.65.9.3942-3949.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knabel SJ, Walker HW, Hartman PA. Inhibition of Aspergillus flavus and selected gram positive bacterial by chelation of essential metal cations by pholyphosphates. Journal of Food Protection. 1991;54(5):360–365. doi: 10.4315/0362-028X-54.5.360. [DOI] [PubMed] [Google Scholar]
- 29.Legeros RZ, Lee Y-K. Synthesis of amorphous calcium phosphates for hard tissue repair using conventional melting technique. Journal of Materials Science. 2004;39(16):5577–5579. [Google Scholar]
- 30.Lee Y, LeGeros RZ. Calcium phosphate glass (CPG): potential as biomaterial for hard-tissue repair. In: Vallet-Regi M, editor. Progress in Biomaterials. Stafa-Zurich: Key Engineering Materials. 2008. pp. 43–72. [Google Scholar]
- 31.Moon HJ, Kim KN, Kim KM, Choi SH, Kim CK, Kim KD, LeGeros RZ, Lee YK. Effect of calcium phosphate glass on bone formation in calvarial defects of Sprague-Dawley rats. Journal of Materials Science-Materials in Medicine. 2006;17(9):807–813. doi: 10.1007/s10856-006-9839-9. [DOI] [PubMed] [Google Scholar]
- 32.Contardo M, Díaz N, Lobos O, Padilla C, Giacaman R. Oral colonization by Streptococcus mutans and its association with the severity of periodontal disease in adults. Revista clínica de periodoncia, implantología y rehabilitación oral. 2011;4:9–12. [Google Scholar]
- 33.Deppe H, Horch H-H, Schrödl V, Haczek C, Miethke T. Effect of 308-nm excimer laser light on peri-implantitis-associated bacteria—an in vitro investigation. Lasers in Medical Science. 2007;22(4):223–227. doi: 10.1007/s10103-007-0441-2. [DOI] [PubMed] [Google Scholar]
- 34.Han YW, Wang X. Mobile Microbiome: Oral Bacteria in Extra-oral Infections and Inflammation. Journal of Dental Research. 2013;92(6):485–491. doi: 10.1177/0022034513487559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koban I, Jablonowski L, Kramer A, Weltmann K-D, Kocher T. Medical Plasma in Dentistry: A Future Therapy for Peri-implantitis. In: Machala Z, Hensel K, Akishev Y, editors. Plasma for Bio-Decontamination, Medicine and Food Security: Springer Netherlands. 2012. pp. 191–200. [Google Scholar]
- 36.Kumar PS, Mason MR, Brooker MR, O'Brien K. Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. Journal of Clinical Periodontology. 2012;39(5):425–433. doi: 10.1111/j.1600-051X.2012.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liljemark WF, Bloomquist C. Human oral microbial ecology and dental caries and periodontal diseases. Critical Reviews in Oral Biology & Medicine. 1996;7(2):180–198. doi: 10.1177/10454411960070020601. [DOI] [PubMed] [Google Scholar]
- 38.Loesche WJ. Role of Streptococcus-mutans in Human Dental Decay. Microbiological Reviews. 1986;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanagawa M, Yoshida K, Matsumoto S, Yamada T, Atsuta M. Inhibitory Effect of Antibacterial Resin Composite against Streptococcus mutans. Caries Research. 1999;33(5):366–371. doi: 10.1159/000016535. [DOI] [PubMed] [Google Scholar]
- 40.Lee JH, Lee C-K, Chang B-S, Ryu H-S, Seo J-H, Hong KS, Kim H. In vivo study of novel biodegradable and osteoconductive CaO-SiO2-B2O3 glass-ceramics. Journal of Biomedical Materials Research Part A. 2006;77A(2):362–369. doi: 10.1002/jbm.a.30594. [DOI] [PubMed] [Google Scholar]
- 41.Allan I, Newman H, Wilson M. Antibacterial activity of particulate bioglass against supra- and subgingival bacteria. Biomaterials. 2001;22(12):1683–1687. doi: 10.1016/s0142-9612(00)00330-6. [DOI] [PubMed] [Google Scholar]
- 42.Dashper SG, O'Brien-Simpson NM, Cross KJ, Paolini RA, Hoffmann B, Catmull DV, Malkoski M, Reynolds EC. Divalent metal cations increase the activity of the antimicrobial peptide kappacin. Antimicrobial Agents and Chemotherapy. 2005;49(6):2322–2328. doi: 10.1128/AAC.49.6.2322-2328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pizzey RL, Marquis RE, Bradshaw DJ. Antimicrobial effects of o-cymen-5-ol and zinc, alone & in combination in simple solutions and toothpaste formulations. International Dental Journal. 61:33–40. doi: 10.1111/j.1875-595X.2011.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai S, Simionato MRL, Mayer MPA, Novo NF, Zelante E. Effects of subinhibitory concentrations of chemical agents on hydrophobicity and in vitro adherence of Streptococcus mutans and Streptococcus sanguis. Caries Research. 1994;28(5):335–341. doi: 10.1159/000261998. [DOI] [PubMed] [Google Scholar]
- 45.Haimi S, Gorianc G, Moimas L, Lindroos B, Huhtala H, Räty S, Kuokkanen H, Sándor GK, Schmid C, Miettinen S, et al. Characterization of zinc-releasing three-dimensional bioactive glass scaffolds and their effect on human adipose stem cell proliferation and osteogenic differentiation. Acta Biomaterialia. 2009;5(8):3122–3131. doi: 10.1016/j.actbio.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Koo H, Sheng J, Nguyen PTM, Marquis RE. Co-operative inhibition by fluoride and zinc of glucosyl transferase production and polysaccharide synthesis by mutans streptococci in suspension cultures and biofilms. FEMS Microbiology Letters. 2006;254(1):134–140. doi: 10.1111/j.1574-6968.2005.00018.x. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen TMP PT, Robert EM. Zinc effects on oxidative physiology of oral bacteria. Advances in Natural Sciences. 2006;7:131. [Google Scholar]
- 48.Oudadesse H, Dietrich E, Gal YL, Pellen P, Bureau B, Mostafa AA, Cathelineau G. Apatite forming ability and cytocompatibility of pure and Zn-doped bioactive glasses. Biomedical Materials. 6(3):035006. doi: 10.1088/1748-6041/6/3/035006. [DOI] [PubMed] [Google Scholar]
- 49.Young RA, Elliott JC. Atomic-scale bases for several properties of apatites. Archives of Oral Biology. 1966;11(7):699–707. doi: 10.1016/0003-9969(66)90095-1. [DOI] [PubMed] [Google Scholar]
- 50.Grynpas MD. Fluoride effects on bone crystals. Journal of Bone and Mineral Research. 1990;5(S1):S169–S175. doi: 10.1002/jbmr.5650051362. [DOI] [PubMed] [Google Scholar]
- 51.Legeros RZ, Kijkowska R, Jia W, Legeros JP. Flouride-cation interactions in the formation and stability of apatites. Journal of Fluorine Chemistry. 1988;41(1):53–64. [Google Scholar]
- 52.Mizrahi B, Shapira L, Domb AJ, Houri-Haddad Y. Citrus Oil and MgCl2 as Antibacterial and Anti-Inflammatory Agents. Journal of Periodontology. 2006;77(6):963–968. doi: 10.1902/jop.2006.050278. [DOI] [PubMed] [Google Scholar]
- 53.Aranha H, Evans SL, Arceneaux JEL, Byers BR. Calcium Modulation of Growth of Streptococcus mutans. Journal of General Microbiology. 1986;132(9):2661–2663. doi: 10.1099/00221287-132-9-2661. [DOI] [PubMed] [Google Scholar]
- 54.Miettinen IT, Vartiainen T, Martikainen PJ. Phosphorus and bacterial growth in drinking water. Applied and Environmental Microbiology. 1997;63(8):3242–3245. doi: 10.1128/aem.63.8.3242-3245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreau JL, Sun LM, Chow LC, Xu HHK. Mechanical and acid neutralizing properties and bacteria inhibition of amorphous calcium phosphate dental nanocomposite. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 98B(1):80–88. doi: 10.1002/jbm.b.31834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Handelman SI, Kreinces GH. Effect of phosphate and pH on Streptococcus mutans acid production and growth. Journal of Dental Research. 1973;52(4):651–657. doi: 10.1177/00220345730520040301. [DOI] [PubMed] [Google Scholar]
- 57.Lee RM, Hartman PA, Stahr HM, Olson DG, Williams FD. Antibacterial mechanism of long-chain polyphosphates in Staphylcoccus aureus. Journal of Food Protection. 1994;57(4):289–294. doi: 10.4315/0362-028X-57.4.289. [DOI] [PubMed] [Google Scholar]
- 58.Loessner MJ, Maier SK, Schiwek P, Scherer S. Long-chain polyphosphates inhibit growth of Clostridium tyrobutyricum in processed cheese spreads. Journal of Food Protection. 1997;60(5):493–498. doi: 10.4315/0362-028X-60.5.493. [DOI] [PubMed] [Google Scholar]
- 59.Obritsch JA, Ryu D, Lampila LE, Bullerman LB. Antibacterial effects of long-chain polyphosphates on selected spoilage and pathogenic bacteria. Journal of Food Protection. 2008;71(7):1401–1405. doi: 10.4315/0362-028x-71.7.1401. [DOI] [PubMed] [Google Scholar]
- 60.Mijares DQ. Synthetic bone mineral (SBM): effect on preventing bone loss induced by estrogen deficiency in a rat model. New York: New York University; 2011. p. 75. [Google Scholar]