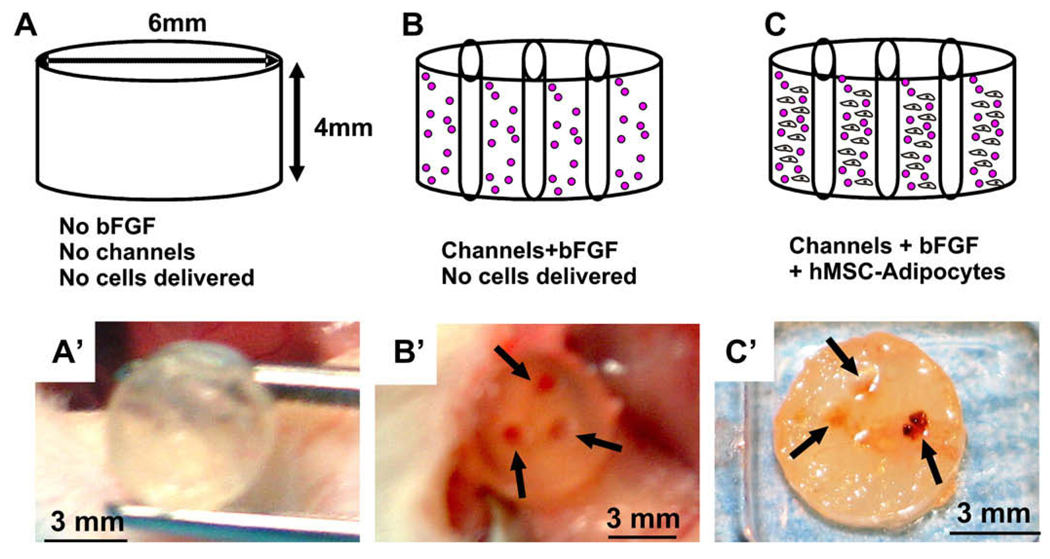
Fig. 2.
In vivo implantation of bFGF and microchanneled PEG hydrogel loaded with adipogenic cells derived from hMSCs. Diagrams (top row) and corresponding representative photographs at the time of harvest of in vivo samples. (A) PEG hydrogel molded into 6 × 4 mm (width × height) cylinder (without either bFGF or microchannels). (B) PEG hydrogel cylinder with 0.5 mg/mL bFGF and three microchannels, but without the delivery of cells. (C) PEG hydrogel cylinder loaded with 0.5 mg/mL bFGF and three microchannels, in addition to the encapsulation of adipogenic cells that have been derived from human mesenchymal stem cells at a cell seeding density of 3 × 106 cells/mL. Following in vivo implantation subcutaneously in the dorsum of immunodeficient mice, the harvested PEG hydrogel samples showed distinct histological features. (A’) PEG hydrogel cylinder without either microchannels or bFGF showed somewhat transparent appearance. (B’) PEG hydrogel cylinder with both bFGF and three microchannels, but without delivered cells, showed darker color and a total of three openings of microchannels (arrows) that are confirmed to be areas of host cell infiltration histologically. (C’) PEG hydrogel cylinder with both microchannels and bFGF in addition to encapsulated hMSC-derived adipogenic cells showed the opening of microchannels (red color and pointed with arrows) that are confirmed to be areas of host cell infiltration histologically in Fig. 3.