Abstract
Hypertrophic cardiomyopathy (HCM) is a common condition defined as a diffuse or segmental left ventricular (LV) hypertrophy with a nondilated and hyperdynamic chamber as well as cardiac arrhythmias. Cardiac MR (CMR) imaging is a key modality for evaluation of HCM. In addition to the assessment of LV wall thickness, LV function and aortic flow, CMR is capable of estimation of late gadolinium enhancement (LGE) in affected myocardium which has been shown to have a direct correlation with incidence and severity of arrhythmias in HCM. In patients with HCM, LGE on CMR is presumed to represent intramyocardial fibrosis. Meanwhile, F-18 FDG myocardial PET has been sporadically studied in HCM, mostly for evaluation of the metabolic status of a hypertrophic myocardial segment, especially after interventions or to demonstrate partial myocardial fibrosis. We presented here the case of a 25-year-old male patient referred for simultaneous F-18 FDG cardiac PET/MR for the evaluation of septal hypertrophy. The PET/MR revealed myocardial fibrosis in the septum associated with FDG-defect and LGE.
Keywords: Hypertrophic cardiomyopathy, Cardiovascular magnetic resonance, FDG, PET/MRI, Fibrosis
Introduction
Hypertrophic cardiomyopathy (HCM) is defined as an unexplained increase of left ventricular wall thickness with a nondilated and hyperdynamic chamber [1]. HCM is the most common genetic cardiovascular disease that is characterized by considerable heterogeneity with regard to manifestation, phenotypic expression, clinical course, and overall prognosis [2]. The clinical course of patients with HCM can be generally benign; however, HCM presents an annual mortality rate of about 1 %, but a subset of roughly 10 % to 20 % of patients have higher mortality or morbidity due to complications arising from sudden cardiac death, progressive heart failure, and atrial fibrillation complicated by embolic stroke [1]. Moreover, risk stratification for the prediction of cardiac death is inaccurate, and the positive predictive value of currently recognized risk markers is low [3].
Contrast-enhanced cardiac MRI (CMR) demonstrates high accuracy in the detection of myocardial infarction and in the differentiation of variant cardiomyopathies [4]. CMR imaging is a noninvasive useful modality for structural and functional evaluations in patients with HCM. In patients with HCM, late gadolinium enhancement (LGE) is presumed to represent intramyocardial fibrosis and has a direct correlation with incidence and severity of arrhythmias in HCM [5].
Positron emission tomography (PET) myocardial perfusion studies have shown slight impairment of myocardial blood flow with pharmacological stress in hypertrophic myocardium presumably related to microvascular disease [6]. F-18 FDG myocardial PET has been sporadically studied in HCM, mostly for evaluation of the metabolic status of the hypertrophic myocardial segment, especially after interventions like transcoronary ablation of septal hypertrophy or to demonstrate partial myocardial fibrosis [7, 8]. Here we report the findings of HCM on integrated simultaneous F-18 FDG myocardial PET and CMR acquisition.
Case Report
A 25-year-old man was referred to cardiology department with incidental ECG abnormality after his left second and fourth fingers were fractured. Although he has not been to a doctor, he has been suffering from mild dyspnea with chest discomfort at rest and exacerbation at exercise since several months ago. Echocardiography revealed nonobstructive HCM with trivial mitral regurgitation. Patient was referred for a simultaneous PET/MR study for F-18 FDG myocardial PET and CMR with gadolinium (Gd) contrast for evaluation of the morphological and metabolic status of the hypertrophic myocardium.
The F-18 FDG study was performed after oral glucose load with 50 g glucose given 1 h prior to injection of 370 MBq F-18 FDG. Simultaneous cardiac PET/MR study performed on an integrated PET/MR scanner (Biograph mMR, Siemens healthcare, Erlangen, Germany) was started 1 h following tracer injection. Following standard Dixon sequence acquisition for attenuation correction, the comprehensive CMR sequences were acquired including steady state free precession cine two-chamber view, three-chamber view, four-chamber view and short-axis images MR perfusion after Gd-contrast infusion and post-contrast LGE studies (Fig. 1). Static F-18 FDG myocardial PET was acquired using list mode for 30 min simultaneously during the MRI acquisition (Fig. 2). Cine MRI and echocardiography showed a nonobstructive form of HCM at rest and normal-sized cardiac chamber with normal LV systolic function. The maximal septal thickness was measured as 30 mm at end diastole on cine MRI, and the first pass MR perfusion showed reduced myocardial blood flow in the hypertrophic septum. The patchy F-18 FDG defect was noted within the hypertrophic septum corresponding to the area of LGE that reflected myocardial fibrosis within the asymmetric septal hypertrophy (Fig. 3). The patient was transferred to another hospital for insertion of an implantable cardioverter defibrillator because he had several risk factors for sudden cardiac death, such as LV maximal wall thickness of 30 mm, presence of fibrosis, and perfusion defect on septum.
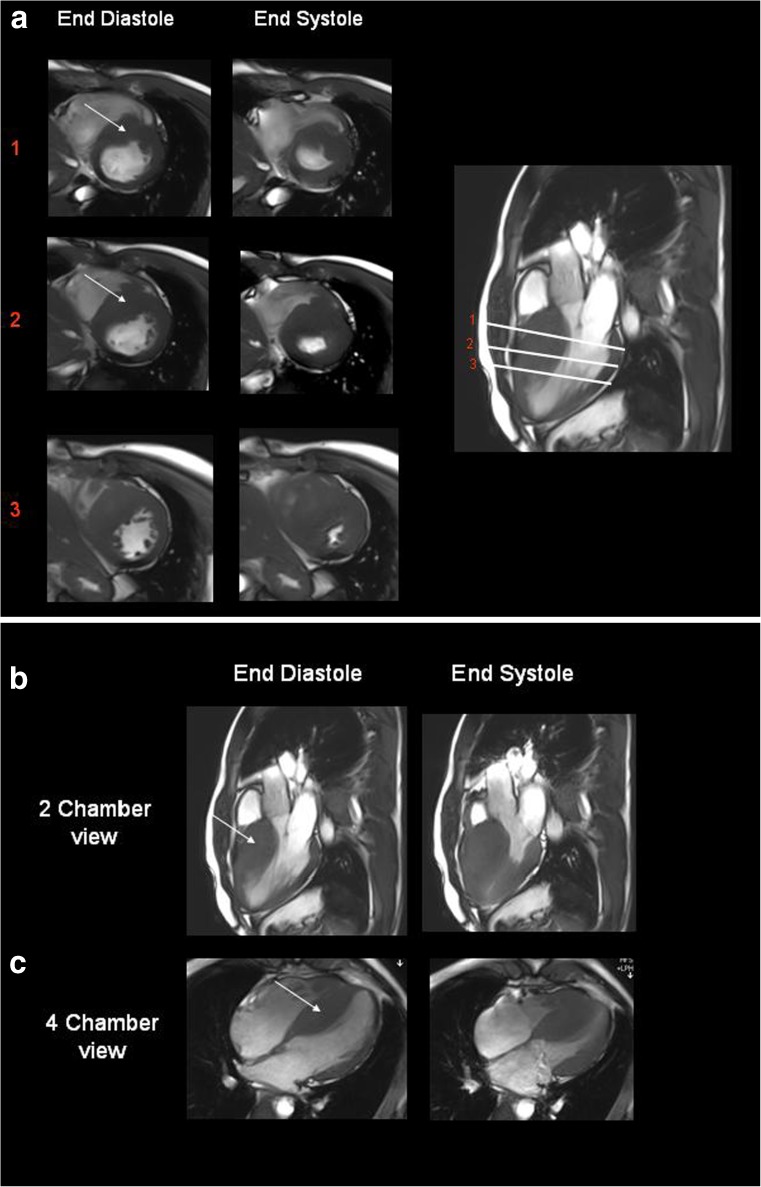
Fig. 1.
The short-axis views (a) of end diastole and end systole at three different sections in the left ventricle was obtained from steady state free precession cine MRI acquisitions performed on the simultaneous PET/MR scanner. The thick hypertrophic septum (arrow) demonstrates the degree of asymmetric septal hypertrophy. End diastolic and end systolic views of two-chamber (b) and four-chamber views(c) obtained from gated cine TruFisp acquisitions show the thickness of asymmetric septal hypertrophy (arrow)
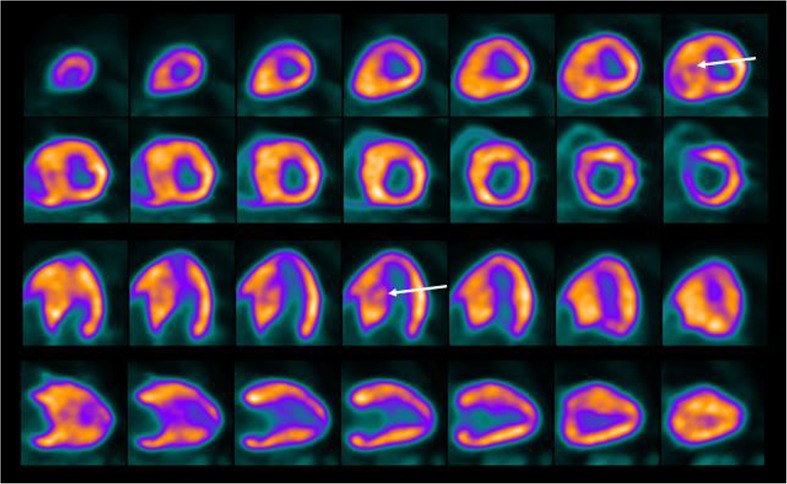
Fig. 2.
Static F-18 FDG myocardial PET images in short axis, horizontal long axis and vertical long axis views demonstrate normal uptake in the LV myocardium except the defect in the hypertrophied septum (arrows). LV cavity size appears normal
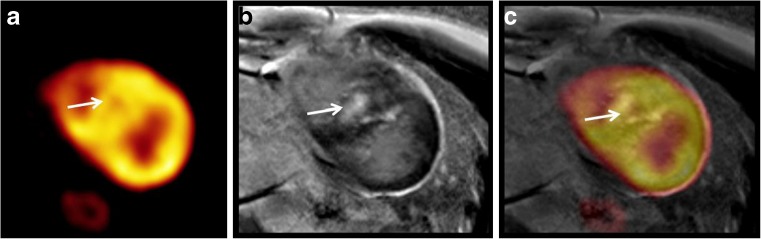
Fig. 3.
Post-contrast CMR (b) short axis images demonstrate late Gd enhancement within the hypertrophied septum (arrow) which shows a corresponding nonuniform patchy defect of F-18 FDG (a, c)
Discussion
HCM is an inherited cardiac disease. Its early detection is important because it is the most common cause of sudden cardiac death among young people. However, HCM is often a challenge for physicians because it presents with diverse phenotypic expressions and clinical courses.
With the advances in MRI, CMR with high spatial resolution and tomographic imaging capability can allow identifying areas of segmental hypertrophy, characterization of the pattern and distribution of LV hypertrophy in HCM. LGE CMR imaging techniques can provide unique information for tissue characterization, specifically for the identification of myocardial fibrosis or scarring [5]. The exact pathophysiologic mechanism of LGE in HCM currently remains unclear. Nevertheless, investigations from recent imaging and histologic studies suggested that LGE was derived from a pathophysiologic cascade in which repetitive encounters of microvascular ischemia resulted from structural abnormalities, such as intramural coronary arteries with impaired vasodilatory capacity. For a certain period, myocardial ischemia-mediated myocyte death finally brings about repair in the form of replacement fibrosis [9]. In addition, based on recent studies, an apparent association has been proved between LGE and ventricular tachyarrhythmias [5, 10]. Up to a seven-fold increased risk for potential lethal ventricular tachyarrythmias has been manifested among HCM patients with LGE compared to those without LGE [10].
However, LGE is not associated with severe symptoms. Several CMR observations in HCM would suggest that not all LGE may represent myocardial fibrosis in HCM, because LGE in HCM is more commonly found in those LV segments which are the thickest with preserved regional systolic function [11]. Extensive amounts of LGE can also be presented in asymptomatic old-age HCM patients (>60 years) with normal systolic function and without adverse disease consequences, such as potentially lethal arrhythmias, heart failure symptoms or adverse LV remodeling [11]. Therefore, some LGE in HCM might not represent myocardial fibrosis. For instance, Gd may deposit within the diffuse interstitial matrix compartment between normally aligned myofibrils or areas of perivascular fibrosis [12]. Current CMR technology cannot distinguish matrix from replacement fibrosis, or an expanded matrix compartment created by myocyte disarray [13].
There are a few studies on HCM using F-18 FDG myocardial PET. Previous studies reported glucose impairment in the affected lesions of patients with apical HCM, and hypertrophic myocardium which showed both oxidative and glucose metabolism impairment seemed to be fibrotic change [14, 15]. HCM showed that impairment of glucose metabolism preceded the reduction of myocardial blood flow [14]. Wang et al. compared the LGE on CMR with F-18 FDG myocardial PET on dilated cardiomyopathy patients and suggested that F-18 FDG myocardial PET could detect more impaired but viable myocardium [16]. Small animal studies showed the F-18 FDG myocardial PET was feasible and useful examination for the repeating assessment of HCM, moreover the reduction of glucose uptake in progressive hypertrophy of myocardium over time may precede its progression to heart failure [17]. Therefore, F-18 FDG defect on hypertrophic myocardium is related with fibrosis, and F-18 FDG myocardial PET shows not only fibrosis as LGE on CMR but also its severity. Consequentially, F-18 FDG myocardial PET imaging contains biologic information and the late stage of hypertrophic myocardium might show more decrease of F-18 FDG uptake. F-18 FDG myocardial PET might be useful to follow up, to evaluate the response by treatment and to estimate prognosis. However, the spatial resolution is better in CMR than F-18 FDG myocardial PET, so integrated PET/MR may be preferable choice.
Simultaneous PET/MR acquisition provides combined acquisition of both modalities, thereby ensuring accurate fusion between morphological and functional images due to simultaneous PET acquisition for every MR sequence. Combination of CMR with F-18 FDG myocardial PET can provide a more detailed risk assessment in cardiomyopathy, and it is also able to give more information about LV function, myocardial perfusion and scar delineation with high soft tissue contrast without ionizing radiation. The good coregistration of the patchy F-18 FDG defect in the area of Gd enhancement within the hypertrophic upper septum reflects the advantage of simultaneous acquisition in our case. By extension, integrated simultaneous PET/MR study could show morphologic and contractile problem and their underlying tissue characterization such as fibrosis with metabolic state in various cardiac disease. In conclusion, F-18 FDG cardiac PET/MR might be helpful in the differential diagnosis of LGE and risk stratification of HCM.
Acknowledgments
Conflict of interest
Neither I nor any co-author have a relevant financial interest/arrangement or affiliation currently or within the past 12 months with the manufacturer of any of the products or provider(s) of any of the services used on patients related to the content of this report.
References
- 1.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 2.Kelley-Hedgepeth A, Maron MS. Imaging techniques in the evaluation and management of hypertrophic cardiomyopathy. Curr Heart Fail Rep. 2009;6:135–141. doi: 10.1007/s11897-009-0020-x. [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ, Spirito P, Shen WK, Haas TS, Formisano F, Link MS, et al. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA. 2007;298:405–412. doi: 10.1001/jama.298.4.405. [DOI] [PubMed] [Google Scholar]
- 4.Jackson E, Bellenger N, Seddon M, Harden S, Peebles C. Ischaemic and non-ischaemic cardiomyopathies–cardiac MRI appearances with delayed enhancement. Clin Radiol. 2007;62:395–403. doi: 10.1016/j.crad.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010;3:51–58. doi: 10.1161/CIRCHEARTFAILURE.109.854026. [DOI] [PubMed] [Google Scholar]
- 6.Bravo PE, Pinheiro A, Higuchi T, Rischpler C, Merrill J, Santaularia-Tomas M, et al. PET/CT assessment of symptomatic individuals with obstructive and nonobstructive hypertrophic cardiomyopathy. J Nucl Med. 2012;53:407–414. doi: 10.2967/jnumed.111.096156. [DOI] [PubMed] [Google Scholar]
- 7.Funabashi N, Nakagawa K, Komuro I. Partial myocardial fibrosis in hypertrophic cardiomyopathy demonstrated by 18F-fluoro-deoxyglucose positron emission tomography and multislice computed tomography. Int J Cardiol. 2006;107:284–286. doi: 10.1016/j.ijcard.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn H, Gietzen FH, Schafers M, Freick M, Gockel B, Strunk-Muller C, et al. Changes in the left ventricular outflow tract after transcoronary ablation of septal hypertrophy (TASH) for hypertrophic obstructive cardiomyopathy as assessed by transoesophageal echocardiography and by measuring myocardial glucose utilization and perfusion. Eur Heart J. 1999;20:1808–1817. doi: 10.1053/euhj.1999.1692. [DOI] [PubMed] [Google Scholar]
- 9.Maron MS, Olivotto I, Maron BJ, Prasad SK, Cecchi F, Udelson JE, et al. The case for myocardial ischemia in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:866–875. doi: 10.1016/j.jacc.2009.04.072. [DOI] [PubMed] [Google Scholar]
- 10.Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1369–1374. doi: 10.1016/j.jacc.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 11.Maron MS, Appelbaum E, Harrigan CJ, Buros J, Gibson CM, Hanna C, et al. Clinical profile and significance of delayed enhancement in hypertrophic cardiomyopathy. Circ Heart Fail. 2008;1:184–191. doi: 10.1161/CIRCHEARTFAILURE.108.768119. [DOI] [PubMed] [Google Scholar]
- 12.Kuribayashi T, Roberts WC. Myocardial disarray at junction of ventricular septum and left and right ventricular free walls in hypertrophic cardiomyopathy. Am J Cardiol. 1992;70:1333–1340. doi: 10.1016/0002-9149(92)90771-P. [DOI] [PubMed] [Google Scholar]
- 13.Kim RJ, Judd RM. Gadolinium-enhanced magnetic resonance imaging in hypertrophic cardiomyopathy: in vivo imaging of the pathologic substrate for premature cardiac death? J Am Coll Cardiol. 2003;41:1568–1572. doi: 10.1016/S0735-1097(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 14.Tadamura E, Tamaki N, Matsumori A, Magata Y, Yonekura Y, Nohara R, et al. Myocardial metabolic changes in hypertrophic cardiomyopathy. J Nucl Med. 1996;37:572–577. [PubMed] [Google Scholar]
- 15.Shiba N, Kagaya Y, Ishide N, Otani H, Takeyama D, Yamane Y, et al. Heterogeneity of myocardial fluoro-18 2-deoxyglucose uptake in patients with apical hypertrophic cardiomyopathy. Jpn Circ J. 1997;61:223–230. doi: 10.1253/jcj.61.223. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Yan C, Zhao S, Fang W. Comparison of 99mTc-MIBI SPECT/18F-FDG PET imaging and cardiac magnetic resonance imaging in patients with idiopathic dilated cardiomyopathy: assessment of cardiac function and myocardial injury. Clin Nucl Med. 2012;37:1163–1169. doi: 10.1097/RLU.0b013e3182708794. [DOI] [PubMed] [Google Scholar]
- 17.Handa N, Magata Y, Mukai T, Nishina T, Konishi J, Komeda M. Quantitative FDG-uptake by positron emission tomography in progressive hypertrophy of rat hearts in vivo. Ann Nucl Med. 2007;21:569–576. doi: 10.1007/s12149-007-0067-2. [DOI] [PubMed] [Google Scholar]